What is the Difference Between Interesterification and Transesterification
Table of Contents
The key difference between interesterification and transesterification is that interesterification is the reaction between an ester and an alcohol to obtain a different ester, whereas transesterification is the reaction between an ester and an alcohol to replace the alkoxy group.
Interesterification is a biochemical process in which a rearrangement occurs in a fatty acid of a fat product. Transesterification can be described as a process useful to modify the structure of an ester.
CONTENTS
1. Overview and Key Difference
2. What is Interesterification
3. What is Transesterification
4. Similarities – Interesterification and Transesterification
5. Interesterification vs Transesterification in Tabular Form
6. Summary – Interesterification vs Transesterification
What is Interesterification?
Interesterification is a biochemical process in which a rearrangement occurs in a fatty acid of a fat product. This typically happens with a mixture of triglycerides. This process is important in the food industry. It involves the breaking and reforming of the ester bonds that are important in connecting the fatty acid chains to the glycerol hubs of the fat molecules. We can perform interesterification using inorganic catalysts that can cause the yielding of chemical interesterification in the industry. If we use enzymes, we can call it enzymatic interesterification.
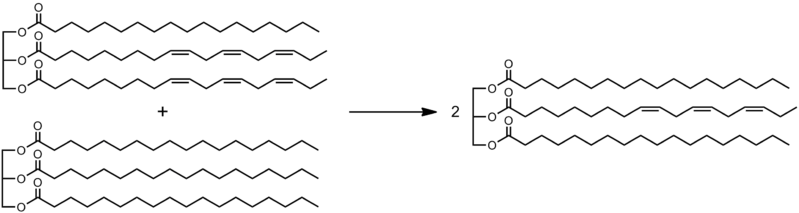
Figure 01: An Example of Interesterification
Generally, the interesterification process can adjust the physical characteristics of the fat product. The properties that it can adjust include melting point, plasticity, etc., for specified applications. For example, we can use interesterification for turning oils into solid or semisolid products by combining the oil with other solid fats.
What is Transesterification?
Transesterification is a process useful to modify the structure of an ester. This process includes an ester and alcohol as reactants. The transesterification process takes place when the alkyl group of an ester is exchanged with the alkyl group of the alcohol. There, the alcohol acts as a nucleophile. The process requires either an acidic catalyst or a basic catalyst. The catalyst can reduce the activation energy barrier of the process.

Figure 02: Transesterification
First, the alcohol is converted into a nucleophile by removing the terminal hydrogen atom as a proton. The transesterification initiates with the nucleophilic attack; alcohol attacks the carbon atom of the ester that is bonded to two oxygen atoms. That is because this carbon atom has a partial positive charge on it since the two oxygen atoms attract the bond electrons toward them (the oxygen atoms are more electronegative than carbon atoms).
The attack by alcoholic nucleophile results in the formation of an intermediate compound that has both the ester and the alcohol bonded with each other through the carbon atom attacked by the nucleophile. This intermediate compound is very unstable. There, a rearrangement occurs to obtain a stable form. This gives a new ester form. Transesterification gives the nucleophile as a byproduct.
What are the Similarities Between Interesterification and Transesterification?
What is the Difference Between Interesterification and Transesterification?
Interesterification and transesterification are related processes that are different from each other according to the objective of the process. The key difference between interesterification and transesterification is that interesterification is the reaction between an ester and an alcohol to obtain a different ester, whereas transesterification is the reaction between an ester and an alcohol to replace the alkoxy group.
Below is a summary of the difference between interesterification and transesterification in tabular form for side by side comparison.
Summary – Interesterification vs Transesterification
Interesterification is a biochemical process in which a rearrangement occurs in a fatty acid of a fat product. Transesterification is a process useful to modify the structure of an ester. The key difference between interesterification and transesterification is that interesterification is the reaction between an ester and an alcohol to obtain a different ester, whereas transesterification is the reaction between an ester and an alcohol to replace the alkoxy group.
Reference:
1. “Transesterification To Biodiesel.” ETIP Bioenergy-SABS.
Image Courtesy:
1. “Interesterification” By Michał Sobkowski – Own work, based on “Margarines and Shortenings” by Ian P. Freeman, Ullmann's Encyclopedia of Industrial Chemistry. 7th Edition 2012; doi:10.1002/14356007.a16_145 (Public Domain) via Commons Wikimedia
2. “Transesterification” (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoyipa2doprAtbHRop2im5GptrC6jJqlnWWkp66vv8Ssq56qmZu2pK3ToqanZw%3D%3D