What is the Difference Between Formalin and Paraformaldehyde
Table of Contents
The key difference between formalin and paraformaldehyde is that formalin is a solution consisting of paraformaldehyde units, whereas paraformaldehyde is a type of polymer formed from formaldehyde.
Formalin is a colorless solution of formaldehyde in the water, while paraformaldehyde is a polymer compound that can be categorized as a poly-acetal.
CONTENTS
1. Overview and Key Difference
2. What is Formalin
3. What is Paraformaldehyde
4. Formalin vs Paraformaldehyde in Tabular Form
5. Summary – Formalin vs Paraformaldehyde
What is Formalin?
Formalin is a colorless solution of formaldehyde in water. It is an organic compound that occurs naturally, and it has the chemical formula CH2O-(H-CHO). Pure formaldehyde has a pungent odor, and it mainly occurs as a smelling colorless gas that can undergo polymerization spontaneously, forming paraformaldehyde.
Formalin can be found in three major forms: buffered, unbuffered, and neutralized forms. These three groups are divided as such based on the buffering capacity of these solutions. These three grades of formalin can be used for studies regarding formalin fixatives. Formalin fixatives are chemical solutions we can use to preserve parts from living things such as animal or plant tissues. The most common formalin fixative used in laboratories is buffered formalin fixative.

Figure 01: Formalin Used to Preserve Animal Tissues
Buffered formalin is the standard and preferred formalin fixative for tissue preservation. This solution is mostly purchased as a prepared solution. Generally, this buffered formalin solution is prepared by mixing one part of stock formalin with nine parts of distilled water. In order to get the buffering capacity, we can add reagents such as monobasic sodium hypophosphate and dibasic or anhydrous sodium hyper phosphate.
Unbuffered formalin is a solution to formalin in water. This type of solution is formed when one part of formalin is mixed with nine parts of water. This solution mixture has a pH of about 3-4, which can vary based on the concentration of the formalin stock we are using for this purpose.
Neutralized formalin is a solution of formalin in water, having a neutral pH value. Therefore, the pH of this type of solution should be 7.0. When formalin stock solution is mixed with water, it gives an acidic solution, so we need to fix the pH using a base such as sodium hydroxide.
What is Paraformaldehyde?
Paraformaldehyde is a polymer compound that can be categorized as a poly-acetal. It is the smallest polyoxymethylene compound. We can obtain this substance as the product of polymerization of formaldehyde with a typical degree of polymerization of 8-100 units. It appears as a crystalline white solid and has an odor resembling formaldehyde. The solubility of this substance in water is very low.
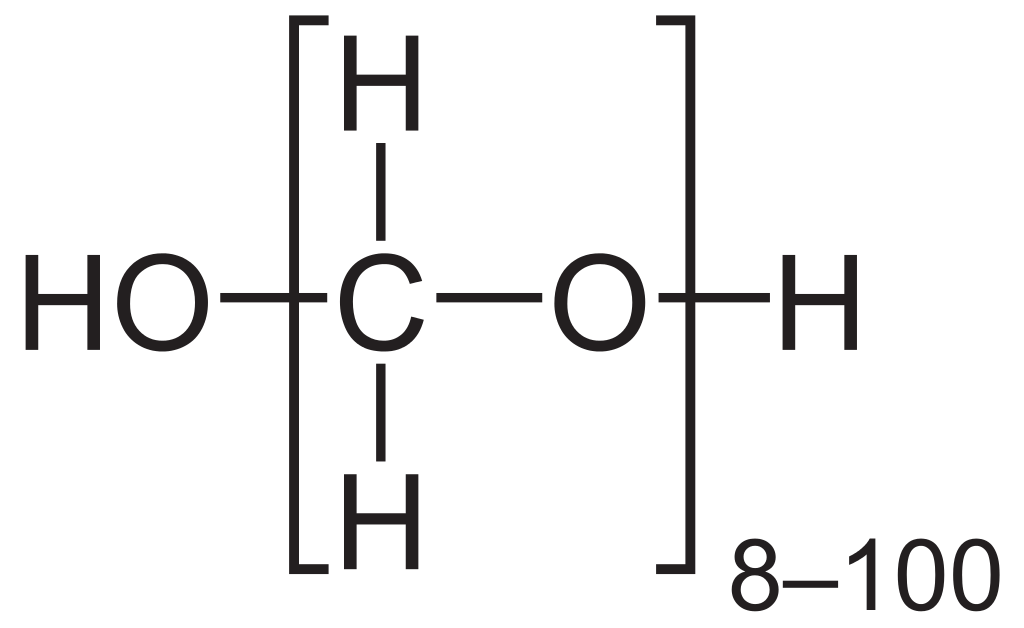
Figure 02: The Chemical Structure of Paraformaldehyde
This substance forms slowly in aqueous formaldehyde solutions in the form of a white precipitate. Specifically, we need to store it in the cold to obtain this precipitate. In contrast, paraformaldehyde can be depolymerized to formaldehyde gas through the dry heating process. This resulting gas is highly flammable.
After the depolymerization, we can use the end product as a fumigant, disinfectant, fungicide, and fixative. If the chain length is considerably long, we can use this compound as a thermoplastic substance as well.
What is the Difference Between Formalin and Paraformaldehyde?
Formalin and paraformaldehyde are related organic compounds. The key difference between formalin and paraformaldehyde is that formalin is a solution consisting of paraformaldehyde units, whereas paraformaldehyde is a type of polymer formed from formaldehyde. Moreover, formalin is useful as an industrial disinfectant, as a preservative in funeral homes and medical labs, while paraformaldehyde is important as a disinfectant, hardening agent, and waterproofing agent.
Below is a summary of the difference between formalin and paraformaldehyde in tabular form for side by side comparison.
Summary – Formalin vs Paraformaldehyde
Formalin is a colorless solution of formaldehyde in water. Paraformaldehyde is a polymer compound that can be categorized as a poly-acetal. The key difference between formalin and paraformaldehyde is that formalin is a solution consisting of paraformaldehyde units, whereas paraformaldehyde is a type of polymer formed from formaldehyde. Therefore, formalin is a collection of polymeric formaldehyde substances.
Reference:
1. “Paraformaldehyde Products” Alfa.com.
Image Courtesy:
1. “Berlin Naturkundemuseum Frogs in Formalin” By LoKiLeCh – Own work (CC BY 3.0) via Commons Wikimedia
2. “Paraformaldehyd” By NEUROtiker – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoyfpqulkaG2r3nAp5tmqJGnrqe70aaYpZyVncalsY4%3D