What is the Difference Between Chemoselectivity and Regioselectivity
Table of Contents
The key difference between chemoselectivity and regioselectivity is that chemoselectivity refers to the preferred reaction of a particular reagent with one, two or more different functional groups, whereas regioselectivity refers to the preference of a chemical bond formation or a chemical bond breaking in one direction over all the other possible directions.
Chemoselectivity and regioselectivity are two different chemical concepts in organic chemistry that are important in describing the properties of chemical reactions. Chemoselectivity is the preferential outcome of a chemical reaction among a set of possible alternative reactions. Regioselectivity in chemistry is the preference of a chemical bond formation or a chemical bond breaking in one direction over all the other possible directions.
CONTENTS
1. Overview and Key Difference
2. What is Chemoselectivity
3. What is Regioselectivity
4. Chemoselectivity vs Regioselectivity in Tabular Form
5. Summary – Chemoselectivity vs Regioselectivity
What is Chemoselectivity?
Chemoselectivity is the preferential outcome of a chemical reaction among a set of possible alternative reactions. It may also refer to the selective reactivity of a particular functional group among other functional groups. This type of prediction depends on the molecular connectivity that is considered to be plausible. However, the physical outcome of this actual reaction tends to be ultimately dependent on a number of factors. Practically, these factors are impossible to predict with any useful accuracy. These factors include solvent, atomic orbitals, etc.
Moreover, chemoselectivity is a difficult property to predict. However, we can commonly observe selective outcomes in some cases that show many reactions that are plausible. A good example of this is selective organic reduction having great relative chemoselectivity of sodium borohydride reduction against the lithium aluminum hydride reaction. Another example involves 4-methoxyacetophenone oxidation by bleach at the ketone group at a high pH value and its oxidation by an aryl chloride at a low PH.
What is Regioselectivity?
Regioselectivity in chemistry is the preference of a chemical bond formation or a chemical bond breaking in one direction over all the other possible directions. This chemical property can be often applied to many possible positions on a molecule where a reagent can affect. For example, protons of an organic molecule that can be abstracted by a strong base, or the location on a substituted benzene ring on which further substitutions can occur.
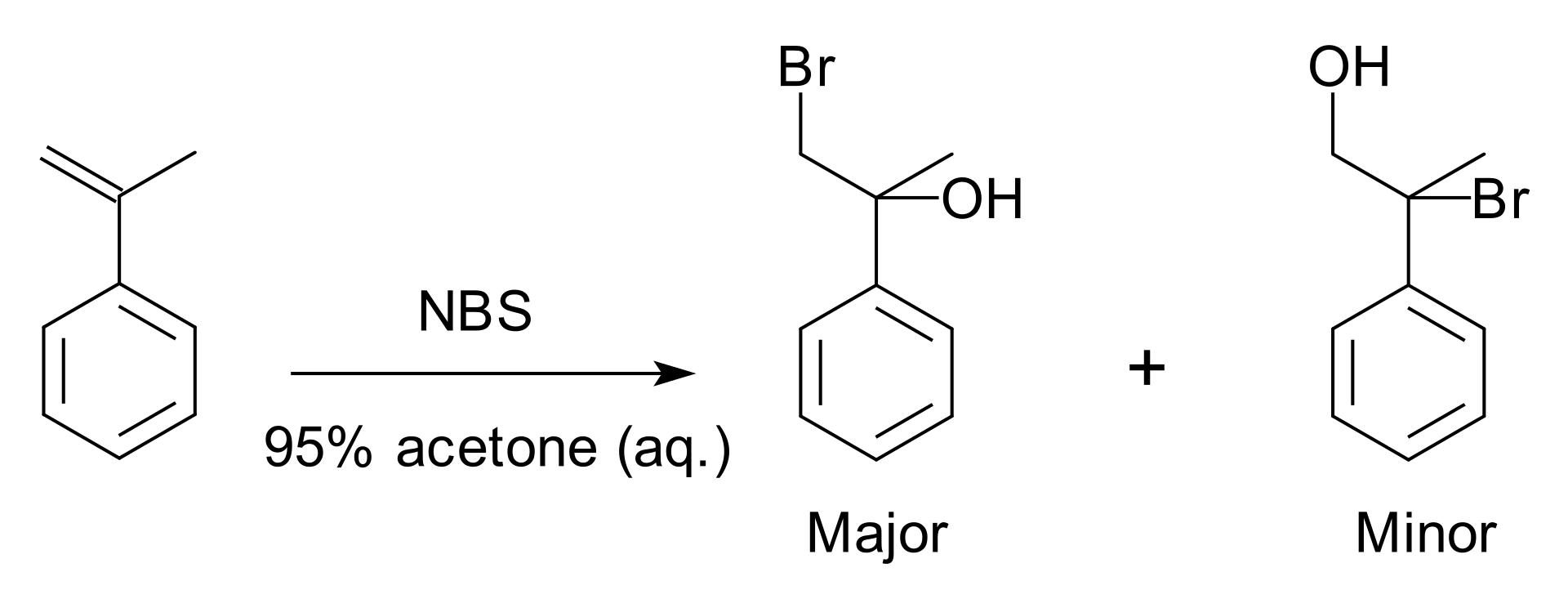
Figure 01: An Example of Regioselectivity
A chemical reaction becomes regioselective when there is a preference for the formation of one product over all other possible products, and this selectivity can generate one constitutional isomer over other forms.
When considering the regioselectivity in ring-closure reactions, these reactions are subjected to Baldwin’s rules. It means if there are more than two orientations for the reaction created during the reaction, one of these orientations is dominant. For example, Markovnikov addition across a double bond.
What is the Difference Between Chemoselectivity and Regioselectivity?
Chemoselectivity and regioselectivity are two different chemical concepts in organic chemistry that are important in describing the properties of chemical reactions. The key difference between chemoselectivity and regioselectivity is that chemoselectivity refers to the preferred reaction of a particular reagent with one, two or more different functional groups, whereas regioselectivity refers to the preference of a chemical bond formation or a chemical bond breaking in one direction over all the other possible directions.
The below infographic lists the differences between chemoselectivity and regioselectivity in tabular form for side by side comparison
Summary – Chemoselectivity vs Regioselectivity
Chemoselectivity and regioselectivity are two different chemical concepts in organic chemistry that are important in describing the properties of chemical reactions. The key difference between chemoselectivity and regioselectivity is that chemoselectivity refers to the preferred reaction of a particular reagent with one, two or more different functional groups, whereas regioselectivity refers to the preference of a chemical bond formation or a chemical bond breaking in one direction over all the other possible directions.
Reference:
1. “9: Chemoselectivity.” Chemistry LibreTexts, Libretexts, 16 Mar. 2021.
Image Courtesy:
1. “RegioselectivityInhalohydrinformation” By V8rik at English Wikipedia (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoycn56ln6iyrbHCraCvoaSueqK6w2apnp%2BZpMCmuMScq6KumanGcA%3D%3D