Difference Between Valence Shell and Penultimate Shell
Table of Contents
The key difference between valence shell and penultimate shell is that valence shell is the outermost electron-containing shell of an atom, whereas penultimate shell is the shell that is inner to the outermost electron-containing shell.
The terms valence shell and penultimate shell are used mainly in general chemistry when determining the electron composition of a particular atom. Valence shell and penultimate shell contain one or more electrons.
CONTENTS
1. Overview and Key Difference
2. What is a Valence Shell
3. What is a Penultimate Shell
4. Side by Side Comparison – Valence Shell vs Penultimate Shell in Tabular Form
5. Summary
What is a Valence Shell?
A valence shell is the outermost electron-containing shell of an atom. The electrons in this shell are called valence electrons. These are the electrons that have the least attraction towards the nucleus of an atom. It is because valence electrons are located a long distance away from the nucleus when compared to the other electrons of that atom.
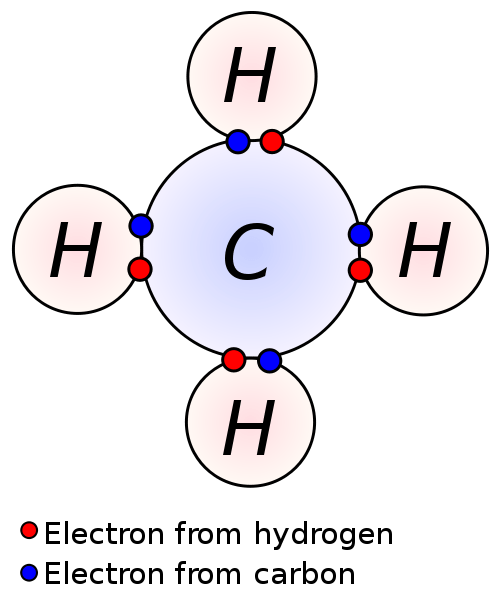
Figure 01: Valence Electrons Involved in Bond Formation
Electrons in the valence shell are responsible for chemical reactions and chemical bondings of atoms. Since the attraction between these electrons and the nucleus of an atom is less, valence electrons can easily be removed (than the electrons in the inner orbitals). This is important in the formation of ionic compounds and covalent compounds. By losing valence electrons, atoms can form cations. Sharing valence electrons of an atom with the valence electrons of another atom result in covalent bonds.
For s block elements and p block elements, the valence shells are s orbitals and p orbitals, respectively. But for transition elements, the valence electrons can be present in inner orbitals as well. This is due to the energy difference between the sub-orbitals. For example, the atomic number of Manganese (Mn) is 25. The electron configuration of cobalt is [Ar] 3d54s2. The valence electrons of cobalt should be in the 4s orbital. But there are 7 valence electrons in Mn. The electrons in the 3d orbital are also considered as valence electrons because the 3d orbital is located outside the 4s orbital (the energy of 3d is higher than the 4s orbital).
What is Penultimate Shell?
Penultimate shell is the electron-containing shell that is inner to the outermost valence shell. In other words, it is the second last electron-filled shell or the shell before the valence shell. Therefore, compared to the valence shell, the penultimate shell has electrons that are more attracted to the atomic nucleus.
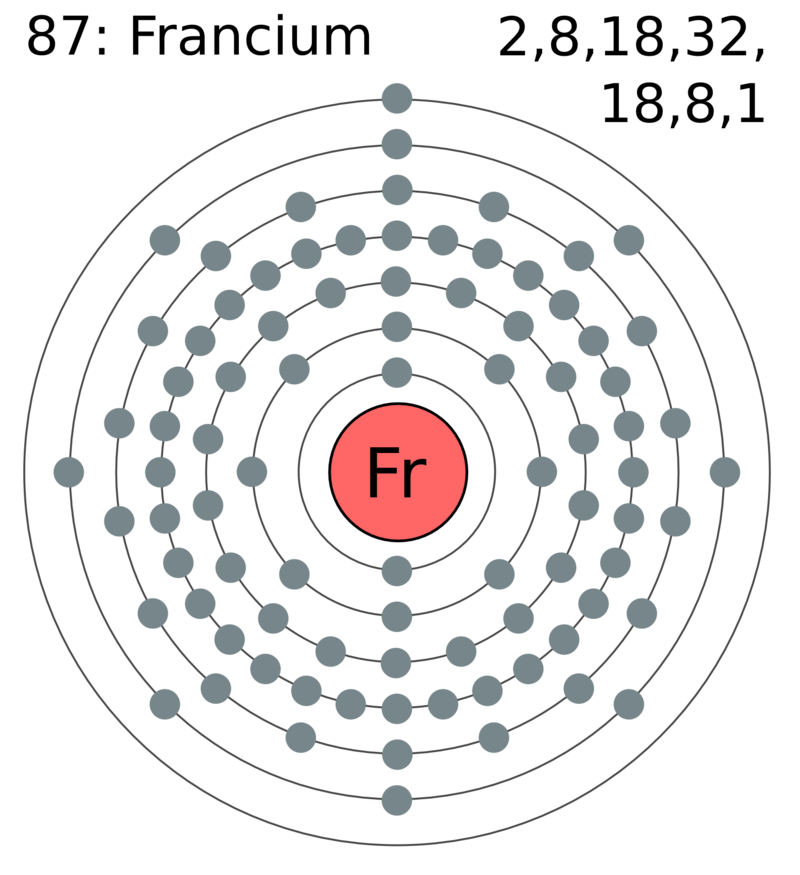
Figure 02: Francium Atom Having Eight Electrons in the Penultimate Shell
Moreover, electrons in the penultimate shell are not involved in the chemical bonding and compound formation processes because they are covered from the valence shell electrons. However, in transition metals, the electrons in the penultimate shell can be the outermost electrons of the metal atom because of the difference in the energies of the sub-orbitals.
What is the Difference Between Valence Shell and Penultimate Shell?
The key difference between valence shell and penultimate shell is that valence shell is the outermost electron-containing shell of an atom. But, penultimate shell is the one inner to the outermost electron-containing shell. Therefore, the penultimate shell is closer to the atomic nucleus than the valence shell.
Moreover, the electrons in the valence shell are less attracted to the atomic nucleus compared to the electrons in the penultimate shell. Apart from that, the electrons in the valence shell are involved in chemical bonding and compound formation, whereas electrons in the penultimate shell are not involved in chemical reactions.
The below infographic tabulates the differences between valence shell and penultimate shell.

Summary – Valence Shell vs Penultimate Shell
Valence shell and penultimate shell are two chemical terms that are very important in general chemistry. The key difference between valence shell and penultimate shell is that valence shell is the outermost electron-containing shell of an atom whereas penultimate shell is the shell that is inner to the outermost electron-containing shell.
Reference:
1. Helmenstine, Anne Marie. “Valence Electron Definition in Chemistry.” ThoughtCo, Feb. 11, 2020, Available here.
2. “Valence Electron.” Wikipedia, Wikimedia Foundation, 6 Feb. 2020, Available here.
Image Courtesy:
1. “Covalent” By DynaBlast – Created with Inkscape (CC BY-SA 2.5) via Commons Wikimedia
2. “Electron shell 087 francium” (CC BY-SA 2.0 UK) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27CwKWcp5uVYsCpsculZJqmlGK9prrUpauipZGpsm6%2Fx56jpWc%3D