Difference Between Urea Formaldehyde and Melamine Formaldehyde
Table of Contents
The key difference between urea formaldehyde and melamine formaldehyde is that urea formaldehyde is made using urea and formaldehyde monomers, whereas melamine formaldehyde is made from the combination of melamine and formaldehyde monomers.
Both urea formaldehyde and melamine formaldehyde are thermosetting polymers that are obtained through irreversibly hardening (also known as curing) a soft solid or a viscous liquid prepolymer.
CONTENTS
1. Overview and Key Difference
2. What is Urea Formaldehyde
3. What is Melamine Formaldehyde
4. Urea Formaldehyde vs Melamine Formaldehyde in Tabular Form
5. Summary – Urea Formaldehyde vs Melamine Formaldehyde
What is Urea Formaldehyde?
Urea formaldehyde or urea-methanal is a non-transparent thermosetting resin or polymer. We can abbreviate it as UF, and it is known as urea formaldehyde because of its common synthesis pathway and overall structure. This resin is important in adhesives, finishes, particleboards, medium density fiberboards, and moulded objects.
The refractive index of urea formaldehyde is 1.55. There are several important properties of urea formaldehyde which include the following:
When considering the chemical structure of urea formaldehyde, it contains [(O)CNHCH2NH]n as the repeating unit. Usually, this resin occurs as chain polymers but depending on the polymerization conditions; there can be branched structures as well. Annually, manufacturers produce about 20 million metric tons of UF. More than 70% of this production is used in the forest-products industry for the bonding particleboard, MDF hardwood plywood, and laminating adhesives.
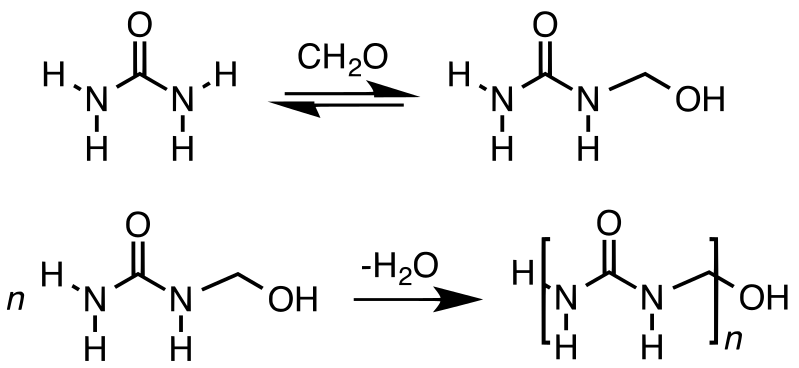
Figure 01: Production of Urea Formaldehyde in Two Steps
This resin is typically pervasive. There are many different applications of urea-formaldehyde, including the production of decorative laminates, textiles, paper, foundry sand moulds, wrinkle-resistant fabrics, cotton blends, rayon, etc. Moreover, there are some agricultural uses of urea-formaldehyde as well, which include its major use as a slow-release source of nitrogen. Furthermore, this resin is used as foam insulation since early times.
What is Melamine Formaldehyde?
Melamine formaldehyde is a resin having melamine rings terminated with multiple hydroxyl groups that are derived from formaldehyde. It is a thermosetting plastic material consisting of melamine and formaldehyde. This resin material was introduced by William F. Talbot in 1936.
This resin material can easily be cured through heating. Here, dehydration and crosslinking occur. We can cure either the melamine-formaldehyde resin or the monomer of this resin material through the treatment with any type of polyol.

Figure 02: A Melamine Formaldehyde Plate
The major application of melamine-formaldehyde resin is the production of high-pressure laminates. The laminate types that we can produce using this resin include Formica and Arborite. Furthermore, we can use the tile wall panels of this resin as whiteboards. In addition, there are some other applications of melamine-formaldehyde resin, including the use of the resin in kitchen utensils and plates. It is also important for saturating decorative paper that is laminated under heat and pressure where it becomes easy to be pasted on particle boards.
What is the Difference Between Urea Formaldehyde and Melamine Formaldehyde?
Urea-formaldehyde and melamine formaldehyde are thermosetting polymers. Urea-formaldehyde or urea-methanal is a non-transparent thermosetting resin or polymer, whereas melamine formaldehyde is a resin having melamine rings terminated with multiple hydroxyl groups that are derived from formaldehyde. The key difference between urea formaldehyde and melamine formaldehyde is that urea-formaldehyde is made using urea and formaldehyde monomers whereas melamine formaldehyde is made from the combination of melamine and formaldehyde monomers.
The following infographic summarizes the difference between urea formaldehyde and melamine formaldehyde in tabular form.
Summary – Urea Formaldehyde vs Melamine Formaldehyde
Urea-formaldehyde and melamine formaldehyde are thermosetting polymers. The key difference between urea formaldehyde and melamine formaldehyde is that urea-formaldehyde is made using urea and formaldehyde monomers whereas melamine formaldehyde is made from the combination of melamine and formaldehyde monomers.
Reference:
1. “Melamine Formaldehyde Resins.” ScienceDirect Topics.
Image Courtesy:
1. “UFresinSyn” By Smokefoot – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Piatto in melammina” By User:Luigi Chiesa – Own work (CC BY 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27B0Z6YZp6fp7qiuMOen7KclWKur7CMppylmZ2eu6Z5xaipppmcmbKpxcOeZg%3D%3D