Difference Between Soluble and Insoluble Salts
Table of Contents
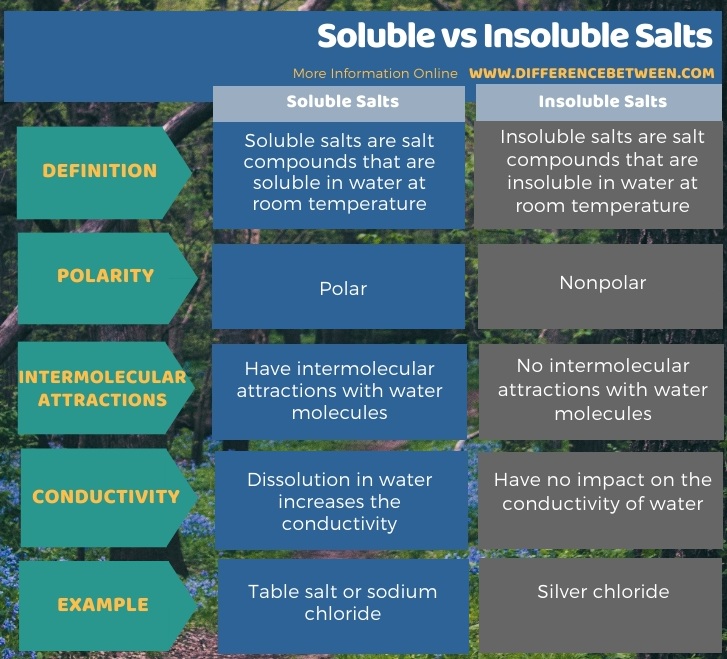
The key difference between soluble and insoluble salts is that soluble salts can dissolve in water at room temperature, whereas insoluble salts cannot dissolve in water at room temperature.
A salt is any compound formed from the reaction between an acid and a base. Therefore, a salt essentially contains an anion (comes from the acid) and a cation (comes from the base). We can divide salt compounds into two types depending on their water solubility at room temperature. They are soluble and insoluble salts. The solubility of salts depends on the types of interactions they can have with water molecules.
CONTENTS
1. Overview and Key Difference
2. What are Soluble Salts
3. What are Insoluble Salts
4. Side by Side Comparison – Soluble vs Insoluble Salts in Tabular Form
5. Summary
What are Soluble Salts?
Soluble salts are salt compounds that are soluble in water at room temperature. These salt compounds dissolve in water because they can form intermolecular attractions with water molecules. Water molecules are polar. Therefore, water is a polar solvent, and polar salts can dissolve in water.

Figure 01: Sodium Chloride is a Soluble Salt
Since salts are ionic compounds, they dissolve in water because water molecules tend to attract the ions in the compound, which makes them separate from each other, resulting in the dissolution of the salt. Here, the dissolution of the salt forms ionic species in water, which makes the newly formed aqueous solution highly conductive. The ionic species dissolved in water can conduct electricity through it. An example of a soluble salt is table salt or sodium chloride. An aqueous solution of table salt contains sodium ions and chloride ions.
What are Insoluble Salts?
Insoluble salts are salt compounds that are insoluble in water at room temperature. These are insoluble in water because water molecules cannot attract the ions in the salt compound. Therefore, there are no intermolecular interactions between water molecules and insoluble salt compounds.

Figure 02: Silver Chloride Precipitate in Water
Furthermore, insoluble salts are nonpolar compounds. Unlike soluble salts, the mixing of insoluble salts with water does not make the solution conductive because the salt does not separate into ions. A good example of an insoluble salt is silver chloride (AgCl).
What is the Difference Between Soluble and Insoluble Salts?
We can divide salt compounds into two types depending on their water solubility. They are soluble and insoluble salts. The key difference between soluble and insoluble salts is that the soluble salts can dissolve in water at room temperature, whereas the insoluble salts cannot dissolve in water at room temperature. Moreover, soluble salts are polar; that’s why they can dissolve in water, which is a polar solvent. In contrast, insoluble salts are nonpolar. So, this is another significant difference between soluble and insoluble salts.
In addition to the above, the water molecules can form intermolecular attractions with the ions of soluble salts, but there are no intermolecular interactions between insoluble salts and water. Furthermore, the dissolution of soluble salts in water makes a highly conductive aqueous solution because the ions dissolved in water can conduct electricity through it. Unlike soluble salts, mixing insoluble salts with water does not make water conductive. Sodium chloride is an example of soluble salts, whereas silver chloride is an example for insoluble salt.
Summary – Soluble vs Insoluble Salts
We can divide salt compounds into two types depending on their water solubility. They are soluble and insoluble salts. The key difference between soluble and insoluble salts is that soluble salts can dissolve in water at room temperature, whereas insoluble salts cannot dissolve in water at room temperature. Moreover, soluble salts are polar; that’s why they can dissolve in water, which is a polar solvent. In contrast, insoluble salts are nonpolar.
Reference:
1. “Insoluble Salts.” Chemistry LibreTexts, Libretexts, 5 June 2019, Available here.
2. “Definition of Insoluble Salts (Precipitates).” Cehmicool Dictionary, Available here.
3. “Insoluble Salts.” Salts-Tripod, Available here.
Image Courtesy:
1. “Sodium chloride” By Chemicalinterest – Own work (Public Domain) via Commons Wikimedia
2. “Silver chloride (AgCl)” By Luisbrudna – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2FzqWsm6SVYq6vsIyipaynnKqvrbGMrJilrKNk