Difference Between Sodium Hypochlorite and Hypochlorous Acid
Table of Contents
The key difference between sodium hypochlorite and hypochlorous acid is that sodium hypochlorite contains a sodium cation and hypochlorite anion, whereas hypochlorous acid contains a proton and hypochlorite anion.
Both sodium hypochlorite and hypochlorous acid contain anions made of oxides of chlorine. Both are inorganic ionic compounds. In these two compounds, anions are similar, but cations are different, which makes them have different chemical and physical properties.
CONTENTS
1. Overview and Key Difference
2. What is Sodium Hypochlorite
3. What is Hypochlorous Acid
4. Similarities Between Sodium Hypochlorite and Hypochlorous Acid
5. Side by Side Comparison – Sodium Hypochlorite vs Hypochlorous Acid in Tabular Form
6. Summary
What is Sodium Hypochlorite?
Sodium hypochlorite is an inorganic ionic compound containing sodium and hypochlorite ions. The chemical formula of this compound is NaOCl. It is the sodium salt of hypochlorous acid. Usually, this compound is unstable, and it may even decompose explosively. However, its pentahydrate form is stable. The chemical formula of pentahydrate form is NaOCl.5H2O. Further, the hydrated form has a pale greenish-yellow colour and occurs as a solid. Although this hydrated form is more stable than the anhydrous form, we have to refrigerate it to keep its stability. Moreover, this compound has a sweet, chlorine-like odour, and its molar mass is 74.44 g/mol.
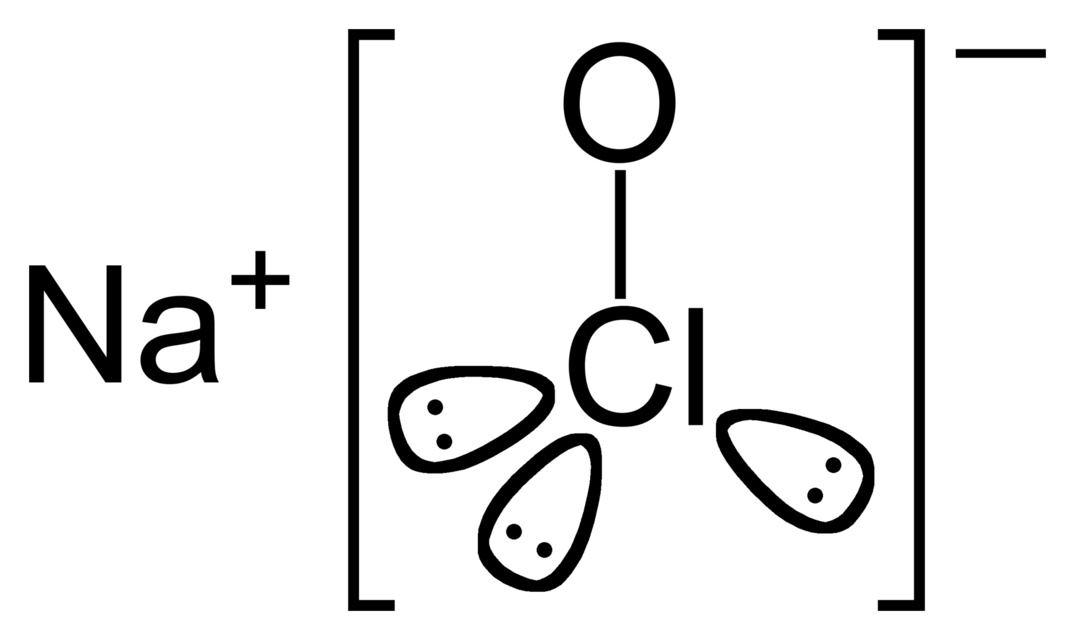
Figure 01: Structure of Sodium Hypochlorite
When considering the preparation methods, we can easily prepare sodium hypochlorite via the reaction between salt (NaCl) and ozone. It is a simple method, but it is suitable for research purposes. For industrial needs, this compound is produced via the Hooker process. In this process, chlorine gas is passed into a dilute sodium hydroxide solution, which gives sodium hypochlorite and sodium chloride.
What is Hypochlorous Acid?
Hypochlorous acid is an inorganic compound having the chemical formula HOCl. It is a weak acid that forms when chlorine gas is dissolved in water. It occurs as a colourless aqueous solution. Its molar mass is 52.46 g/mol.
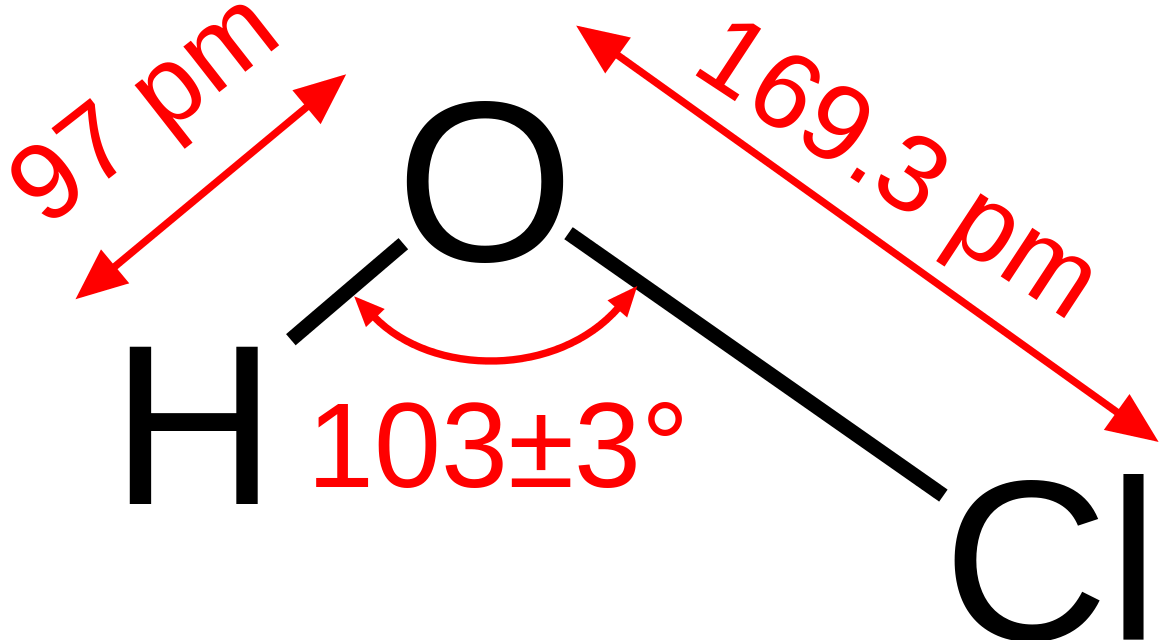
Figure 02: Structure of Hypochlorous Acid
The applications of this weak acid include the followings:
- In organic synthesis as an intermediate
- As an ingredient in cosmetics
- As a disinfectant in foodservice and water distribution processes
- Present in neutrophils and important for the destruction of bacteria
What are the Similarities Between Sodium Hypochlorite and Hypochlorous Acid?
- Sodium hypochlorite and hypochlorous acid contain anions made of oxides of chlorine.
- This anion is hypochlorite anion.
- Both are inorganic ionic compounds.
What is the Difference Between Sodium Hypochlorite and Hypochlorous Acid?
The key difference between sodium hypochlorite and hypochlorous acid is that the sodium hypochlorite contains a sodium cation and hypochlorite anion, whereas the hypochlorous acid contains a proton and hypochlorite anion. Moreover, sodium hypochlorite appears as a pale greenish-yellow solid while hypochlorous acid appears as a clear aqueous solution. Furthermore, we can produce sodium hypochlorite via Hooker process or by the reaction between salt and ozone; in contrast, we can produce hypochlorous acid via dissolution of chlorine gas in water.
When considering the uses of each compound, sodium hypochlorite is useful for the purposes of bleaching, cleaning, disinfection, deodorizing, etc. while hypochlorous acid is important as an intermediate in organic synthesis processes, ingredient in the cosmetics industry, etc.
The below infographic summarizes the difference between sodium hypochlorite and hypochlorous acid.
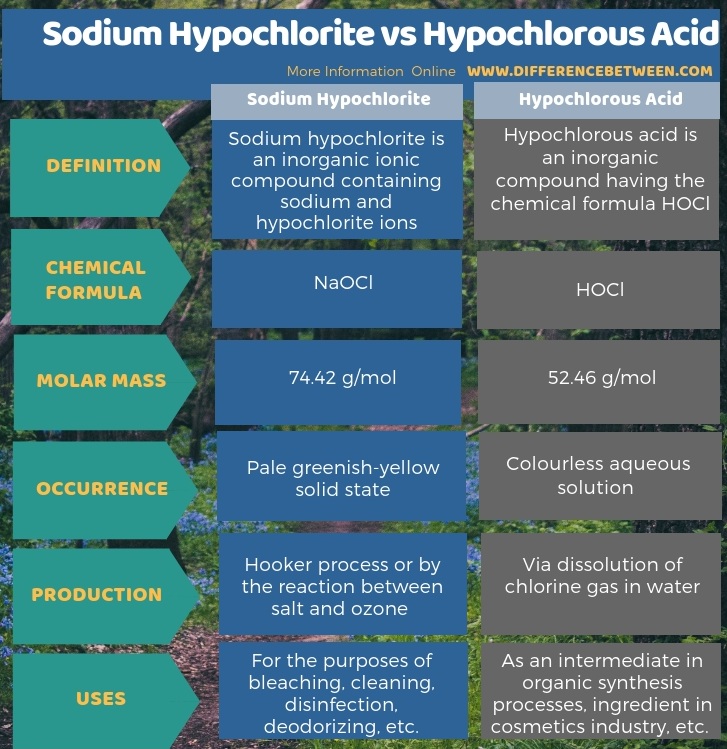
Summary – Sodium Hypochlorite vs Hypochlorous Acid
Sodium hypochlorite and hypochlorous acid contain hypochlorite anions, which are anions made of oxides of chlorine. The key difference between sodium hypochlorite and hypochlorous acid is that sodium hypochlorite contains a sodium cation and hypochlorite anion, whereas hypochlorous acid contains a proton and hypochlorite anion.
Reference:
1. Helmenstine, Anne Marie. “Bleach Facts (Answers to Common Questions).” ThoughtCo, Sep. 21, 2019, Available here.
2. Helmenstine, Anne Marie. “How Does Bleach Work?” ThoughtCo, Sep. 3, 2019, Available here.
Image Courtesy:
1. “Sodium-hypochlorite” By Benjah-bmm27 – Own work, Public Domain) via Commons Wikimedia
2. “Hypochlorous-acid-2D-dimensions” By Liaocyed – Own work (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2Fzp2grqVdncaxu8Kho6iqmamybq3NnWShsaCksKm4zqumrqtdlrCqsI4%3D