Difference Between Quaternary Ammonium and Ammonia
Table of Contents
The key difference between quaternary ammonium and ammonia is that quaternary ammonia molecule has a central nitrogen atom bound to four alkyl groups whereas ammonia molecule contains one nitrogen centre bound to three hydrogen atoms.
Quaternary ammonium is a cation that is derived from a normal ammonia molecule. Here, the three hydrogen atoms of the ammonia molecule are substituted with similar or different alkyl groups, and there is an extra alkyl group bound to the nitrogen atom via its lone electron pair.
CONTENTS
1. Overview and Key Difference
2. What is Quaternary Ammonium
3. What is Ammonia
4. Side by Side Comparison – Quaternary Ammonium vs Ammonia in Tabular Form
5. Summary
What is Quaternary Ammonium?
Quaternary ammonium is a cation derived from ammonia molecule, and it has a central nitrogen atom with four alkyl groups substituted to it. Therefore, the chemical formula of this molecule can be written as [N-R1R2R3R4]+. The chemical structure of this cation is as follows:
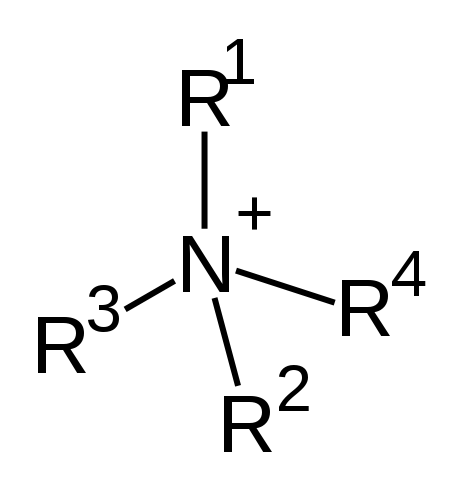
Figure 01: Chemical Structure of Quaternary Ammonium Cation
These cations are abbreviated as quats. They are positively charged polyatomic ions. There are several ions formed by ammonia molecule including primary, secondary and tertiary ammonia. These are named according to the number of alkyl groups bound to the nitrogen atom.
The most common compounds of quaternary ammonium are quaternary ammonium salts. These compounds can be prepared by alkylation of tertiary amines in the presence of halocarbons. Generally, quaternary ammonium cations are unreactive towards even strong oxidants, strong acids, and electrophiles. However, these cations undergo degradation in the presence of exceptionally strong bases.
What is Ammonia?
Ammonia is an inorganic compound having the chemical formula NH3. It is a gaseous substance, and it is the simplest pnictogen hydride. Ammonia occurs as a colourless gas having a pungent, irritating odour. The IUPAC name of Ammonia is azane.
The chemical formula is NH3. Therefore, the molar mass is 17.03 g/mol. The melting point of ammonia is −77.73 °C, and the boiling point is −33.34 °C.
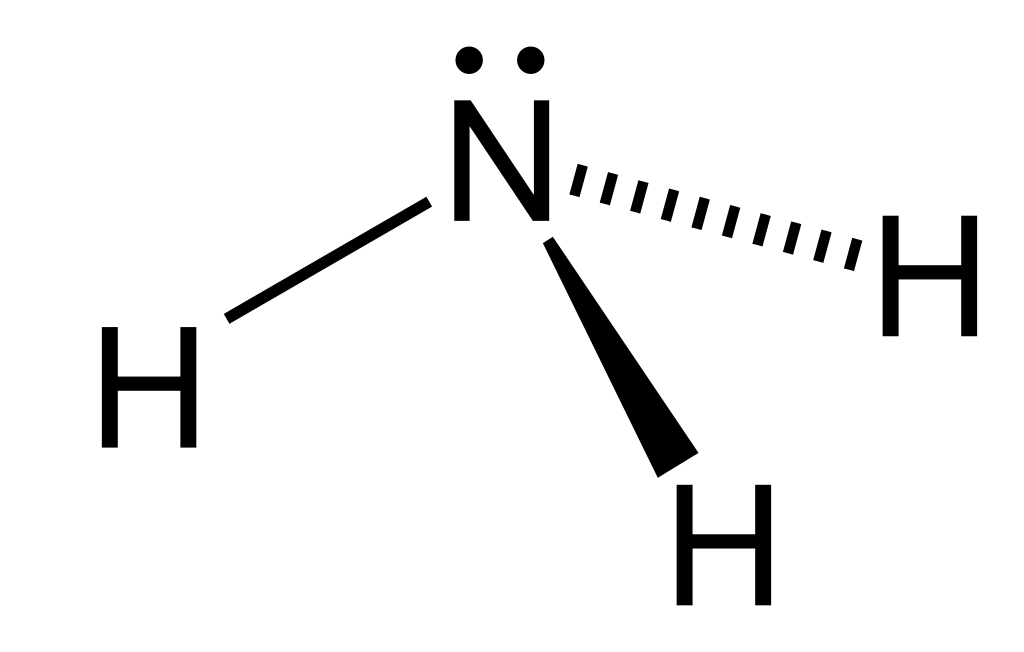
Figure 02: Structure of Ammonia Molecule
When considering the occurrence of ammonia gas, it naturally occurs in the environment but in trace amounts as a product of nitrogenous animal and vegetable matter. Sometimes, we can find ammonia in rainwater as well. Inside our body, kidneys secrete ammonia to neutralize excess acid.
In the chemical structure of the ammonia molecule, it has a nitrogen atom bound to three hydrogen atoms. Since there are five electrons in the outermost electron shell of nitrogen, there is a lone electron pair on the nitrogen atom of the ammonia molecule. Hence, the geometry of the ammonia molecule is trigonal pyramidal. Furthermore, we can liquefy this compound easily. This is because it is capable of forming hydrogen bonds between ammonia molecules since there are N-H bonds and lone electron pairs as well.
What is the Difference Between Quaternary Ammonium and Ammonia?
Quaternary ammonium is a cation derived from an ammonia molecule. The key difference between quaternary ammonium and ammonia is that quaternary ammonia molecule has a central nitrogen atom bound with four alkyl groups whereas ammonia molecule contains one nitrogen centre bound to three hydrogen atoms.
The following table summarizes the difference between quaternary ammonium and ammonia.
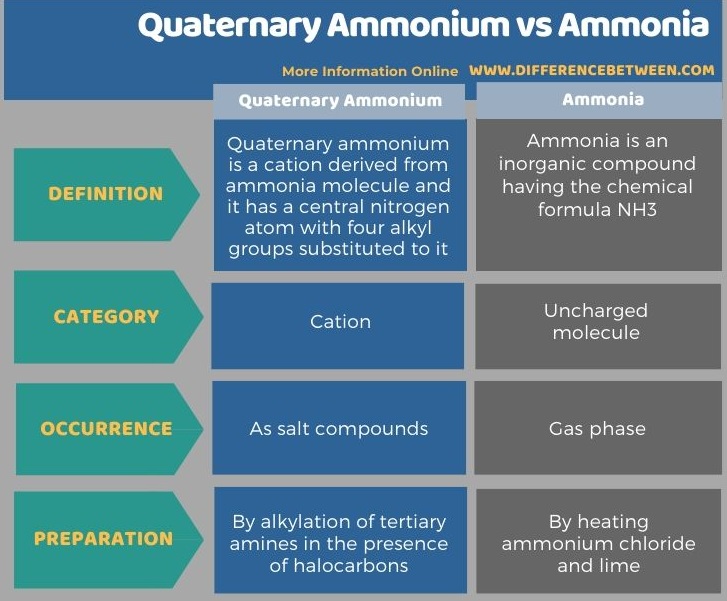
Summary – Quaternary Ammonium vs Ammonia
Quaternary ammonium is a cation derived from an ammonia molecule. The key difference between quaternary ammonium and ammonia is that quaternary ammonia molecule has a central nitrogen atom bound with four alkyl groups whereas ammonia molecule contains one nitrogen centre bound to three hydrogen atoms.
Reference:
1. “Quaternary Ammonium Compounds.” Quaternary Ammonium Compounds – an Overview | ScienceDirect Topics, Available here.
Image Courtesy:
1. “Ammonia-2D” By Radio89 – Own work (CC BY-SA 3.0) via Commons Wikimedia
2. “Quaternary ammonium cation” By Fvasconcellos – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2691Jqrnqqelr%2B6ecCmpKimmaq6bq3NnWSapZ2ku6qtjg%3D%3D