Difference Between Prosthetic Group and Coenzyme
Table of Contents
Key Difference – Prosthetic Group vs Coenzyme
Enzymes are the biological catalysts of chemical reactions occurring in living cells. Some enzymes require helper molecules or partner molecules to catalyze biochemical reactions. These molecules are known as cofactors. Cofactors are non-protein molecules which assist chemical reactions to proceed. They assist in increasing the rate of the reaction. Cofactors can be either inorganic or organic. They are composed of various types of molecules such as vitamins, metal ions, non-vitamin molecules, etc. Prosthetic group and coenzyme are two types of helper molecules of enzymes. The key difference between prosthetic group and coenzyme is that prosthetic group tightly binds with the enzyme to assist enzyme while coenzyme loosely binds with an enzyme to support its catalytic function. Prosthetic groups can be organic molecules or metal ions while coenzymes are totally organic molecules.
CONTENTS
1. Overview and Key Difference
2. What is a Prosthetic Group
3. What is a Coenzyme
4. Side by Side Comparison – Prosthetic Group vs Coenzyme
5. Summary
What is a Prosthetic Group?
A prosthetic group is a cofactor which binds tightly to the enzyme and assists in catalyzing the chemical reaction. They are non-protein molecules. They can be small organic molecules or metal ions. Due to the tight binding to the enzyme, prosthetic groups are difficult to remove from the enzymes. Hence, it is considered that the bond between prosthetic group and enzyme is permanent unlike in coenzymes. Upon binding, they can act as structural elements or as charge carriers. For example, prosthetic group heme in hemoglobin and myoglobin allows binding and releasing of oxygen as per the requirement of tissues. There are some vitamins which act as prosthetic groups for enzymes.
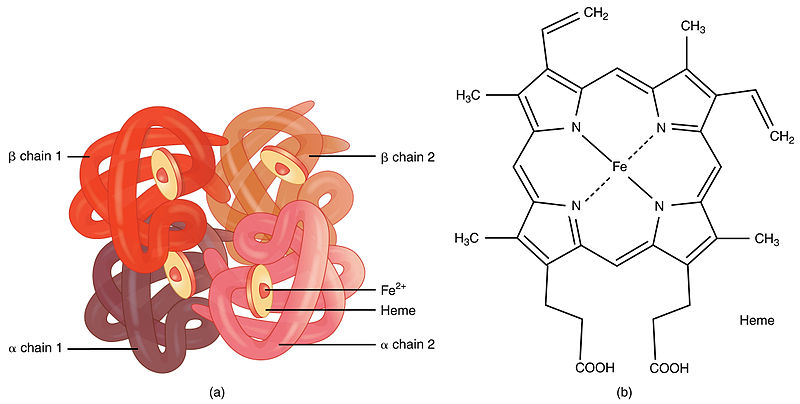
Figure 01: Prosthetic group heme in hemoglobin molecule
What is a Coenzyme?
Coenzyme is a specific type of cofactor which assists enzymes in performing their function. They are involved in increasing the rate of the reaction. Coenzymes are non-protein compounds which work with enzymes. They are small organic molecules (carbon-containing molecules) mostly derived from vitamins. They bind loosely with the active site of the enzyme and help them in recognizing, attracting and repulsing substrates. The presence of a coenzyme is essential for some enzymes to initiate and carry out the catalytic function. They act as intermediate carriers and cosubstrates as well.
Coenzymes are not specific for enzymes. They can bind with many different types of enzymes and aid them to perform chemical reactions. Hence, they are reusable. These coenzymes may change their structures into alternative forms when it is essential. Coenzymes cannot work alone. They should bind with the enzyme. When a coenzyme bind with the apoenzyme it becomes a holoenzyme which is the active form of the enzyme that catalyzes the chemical reactions.
Examples of coenzymes include vitamin C, vitamin B, S-adenosyl methionine, ATP, coenzyme A, etc.

Figure 02: Coenzyme
What is the difference between Prosthetic Group and Coenzyme?
Prosthetic Group vs Coenzyme | |
| Prosthetic group is a type of a helper molecule which is a nonproteinaceous compound that helps enzymes to perform their functions. | Coenzyme is a specific kind of cofactor molecule which is an organic molecule that helps enzymes to catalyze chemical reactions. |
| Bond with Enzymes | |
| They bind tightly or covalently with enzymes to aid enzymes. | They bind loosely with the active site of the enzyme to help catalytic function. |
| Composition | |
| Prosthetic groups are metal ions, vitamins, lipids, or sugars. | Coenzymes are vitamins, vitamin derivatives or nucleotides. |
| Main Function | |
| Prosthetic group mainly provides a structural property to the enzyme. | Coenzyme mainly provides a functional property to the enzyme. |
| Removal from the Enzyme | |
| Prosthetic groups cannot be easily removed from the enzymes. | Coenzymes can be easily removed from the enzymes. |
| Examples | |
| Examples include flavin nucleotides and heme. | Examples include AMP, ATP, coenzyme A, FAD, and NAD+, S-adenosyl methionine |
Summary – Prosthetic Group vs Coenzyme
Cofactors are the helper molecules of enzymes. They are not proteins and are either inorganic or organic molecules. Coenzymes and prosthetic groups are two types of helper molecules. A coenzyme is an organic molecule which binds loosely with enzymes to help reactions. A prosthetic group is an organic molecule or a metal iron which binds tightly or covalently with the enzyme to assist chemical reactions. This is the difference between prosthetic group and coenzyme. Both groups are reusable and nonspecific to the enzymes.
Reference:
1. “Cofactor (biochemistry).” Wikipedia. Wikimedia Foundation, 14 May 2017. Web. 17 May 2017. <https://en.wikipedia.org/wiki/Cofactor_(biochemistry)>.
2. Klucevsek, Kristin. “Coenzymes, Cofactors & Prosthetic Groups: Function and Interactions.” Study.com. Study.com, n.d. Web. 17 May 2017. http://study.com/academy/lesson/coenzymes-cofactors-prosthetic-groups-function-and-interactions.html
3. Helmenstine, Ph.D. Anne Marie. “What Is a Coenzyme? Definition and Examples.” ThoughtCo. N.p., n.d. Web. 17 May 2017. <https://www.thoughtco.com/definition-of-coenzyme-and-examples-604932>.
4. Hashim, Onn H., and Nor Azila Adnan. “Coenzyme, cofactor and prosthetic group — Ambiguous biochemical jargon.” Biochemical Education. Headington Hill Hall, 30 June 2010. Web. 17 May 2017
Image Courtesy:
1. “1904 Hemoglobin” By OpenStax College – Anatomy & Physiology, Connexions Web site. Jun 19, 2013. (CC BY 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2680aiqraCVqbakecarpq6oXZa7pXnVrGScp5Wjx7q5xGg%3D