Difference Between pKa and pKb
Table of Contents
Key Difference – pKa vs pKb
pKa and pKb are common terms in chemistry that are known as dissociation constants. pKa is acid dissociation constant, and pKb is base dissociation constant. These terms are used to make it easy to work with very large or very small values. The “p” in these terms stands for “negative logarithm”. The key difference between pKa and pKb is that pKa is the negative logarithm of Ka whereas pKb is the negative logarithm of Kb.
CONTENTS
1. Overview and Key Difference
2. What is pKa
3. What is pKb
4. Relationship Between pKa and pKb
5. Side by Side Comparison – pKa vs pKb in Tabular Form
6. Summary
What is pKa?
pKa is the negative logarithm of Ka. Ka is the acid dissociation constant of a solution. It is a quantitative measurement of the strength of an acid in a solution. Acids are chemical compounds that can release one or more hydrogen ions (protons) to a solution. If the acid dissociation constant; Ka is higher, it means that acid is completely (or nearly completely) dissociated into ions forming hydrogen ions. Then, it indicates that acid is a strong acid. Since the pKa is the negative logarithmic value of Ka, pKa is a smaller value for strong acid.
pKa = -log10Ka
Lower the pKa vlaue, the stronger the acid is. Similarly, higher the pKa value, the weaker the acid is. By looking at the pKa values of different acids, one can compare the relative acid strengths. Instead of using Ka values, the pKa values are used in common because it makes easier to work with very large or very small numbers with small decimal places.
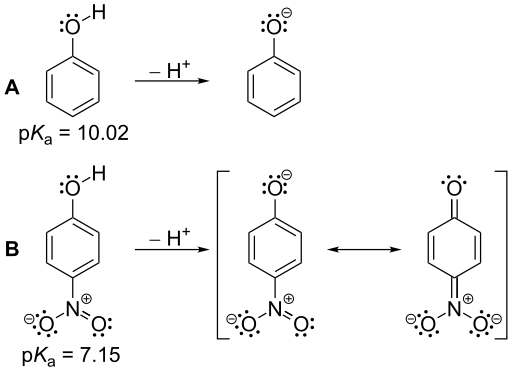
Figure 01: The pKa values of phenol and nitrophenol: nitrophenol is a stronger acid than phenol because of its smaller pKa value compared to nitrophenol.
Apart from comparing acid strength, pKa values are also used to select suitable buffers. According to the Henderson-Hasselbalch Equation, there is a relationship between pH and pKa of a system.
pH = pKa + log10([A–]/[AH])
for the dissociation of HA acid. This equation can be re-written as below.
Ka/[H+] = [A–]/[AH]
According to this equation, the pKa and pH values of the same system are equal when half of the acid has been dissociated. Since the buffering capacity of a system is its ability to maintain the pH of a solution, the buffer should be selected in which the pKa and pH are very close to each other.
What is pKb?
pKb is the negative logarithm of Kb. Kb is the base dissociation constant. It is used to determine the strength of a base quantitatively. When a base is dissolved in water, it dissociates into ions forming a basic solution. Strong bases dissociate completely. Weak bases dissociate partially.
pKb = -log10Kb
The “p” in pKb stands for “negative logarithm”. Since most of the Kb values are very large or very small, negative logarithms of these values are used to make it easy to deal with. Therefore, a large Kb value can be characterized by a small pKb value with small decimal places.
What is the Relationship Between pKa and pKb?
The relationship between Ka and Kb is given as below.
Kw = Ka.Kb
Then the relationship between pKa and pKb is given as, (at 25oC)
pKa + pKb = 14
What is the Difference Between pKa and pKb?
pKa vs pKb | |
| pKa is the negative logarithm of Ka. | pKb is the negative logarithm of Kb. |
| Nature | |
| pKa is given for acids. | pKb is given for bases. |
| Relationship With Dissociation Constant | |
| pKa is related to acid dissociation constant. | pKb is related to the base dissociation constant. |
| Indications | |
| Smaller the pKa value, stronger the acid. | Smaller the pKb value, weaker the base. |
Summary – pKa vs pKb
pKa and pKb are used to compare the strength of acids and bases respectively. pKa is given for acid dissociations. pKb is given for dissociation of bases. The difference between pKa and pKb is that pKa is the negative logarithm of Ka whereas pKb is the negative logarithm of Kb.
Download the PDF of pKa vs pKb
You can download the PDF version of this article and use it for offline purposes as per citation note. Please download the PDF version here: Difference Between pKa and pKb
Reference:
1.Helmenstine, Anne Marie, D. “pKa Definition in Chemistry.” ThoughtCo, Sep. 15, 2017. Available here
2.Helmenstine, Anne Marie, D. “pH and pKa Relationship: The Henderson-Hasselbalch Equation.” ThoughtCo, Dec. 1, 2017. Available here
3.“Relationship between Ka and Kb.” Khan Academy. Available here
Image Courtesy:
1.’PKa phenol vs nitrophenol’By Hbf878 – Own work, (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau268yppkmqaUYsO0ec%2BkmWg%3D