Difference Between Phenol and Phenyl
Table of Contents
The key difference between phenol and phenyl is that phenol has a hydroxyl group whereas phenyl has no hydroxyl group.
Phenol is an aromatic alcohol. It has the chemical formula C6H5OH. Therefore, the chemical structure of the phenol molecule has a benzene ring and a hydroxyl group (-OH) attached to it. Phenyl is a derivative of phenol; if we remove the hydroxyl group from the phenol molecule, it gives phenyl group.
CONTENTS
1. Overview and Key Difference
2. What is Phenol
3. What is Phenyl
4. Side by Side Comparison – Phenol vs Phenyl in Tabular Form
5. Summary
What is Phenol?
Phenol is an aromatic hydrocarbon compound having the chemical formula C6H5OH. This compound exists as a white crystalline solid at room temperature. And also it is a volatile hydrocarbon. When considering the chemical structure of the molecule, it contains a phenyl group attached with a hydroxyl group (-OH). Moreover, this compound is mildly acidic. Thus, we need to take care when handling it.

Figure 01: Chemical Structure of Phenol
The molar mass of this compound is 94.11 g/mol. It has a sweet odour. Furthermore, its melting point and boiling point are 40.5 °C and 181.7 °C respectively. It is miscible with water because it can form hydrogen bonds with water molecules. When it loses the hydrogen of the –OH group it forms the negatively charged phenolate ion, it becomes resonance stabilized, which in turn makes phenol a relatively good acid. In the resonance stabilization, the negative charge on the oxygen atom is shared with the carbon atoms in the ring.
What is Phenyl?
Phenyl is a group of atoms with the formula C6H5. This group derives from benzene, therefore, has similar properties as benzene. However, this differs from benzene due to lack of a hydrogen atom in one carbon. So the molecular weight of phenyl is 77 g mol-1. We can denote phenyl as “Ph”. Usually, phenyl attaches with another phenyl group, atom or molecule (we name this additional part as the substituent).
The carbon atoms of phenyl are sp2 hybridized carbon atoms same as in benzene. All the carbons can form three sigma bonds. Two of the sigma bonds forms between two adjacent carbons so that it will give rise to a ring structure. The other sigma bond forms with a hydrogen atom. However, in phenyl, in one carbon in the ring, the third sigma bond forms with another atom or molecule rather than with a hydrogen atom.
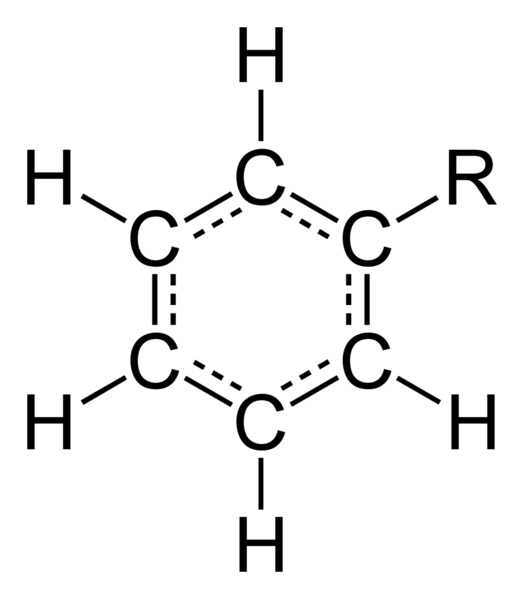
Figure 02: Phenyl Group attached with the Substituent R
The electrons in p orbitals overlap with each other to form the delocalized electron cloud. Therefore, phenyl has similar C-C bond lengths between all carbons, regardless of having alternating single and double bonds. This C-C bond length is about 1.4 Å. The ring is planar and has 120o angle between bonds around a carbon. Due to the substituent group of phenyl, the polarity and other chemical or physical properties change.
If the substituent donates electrons to the delocalized electron cloud of the ring, we name them as electron donating groups. (E.g., – OCH3, NH2) In contrast, if the substituent attracts electrons from the electron cloud, we name them as electron withdrawing substituents. (E.g., -NO2, -COOH). Phenyl groups are stable due to their aromaticity, so they don’t easily undergo oxidations or reductions. Further, they are hydrophobic and non-polar.
What is the Difference Between Phenol and Phenyl?
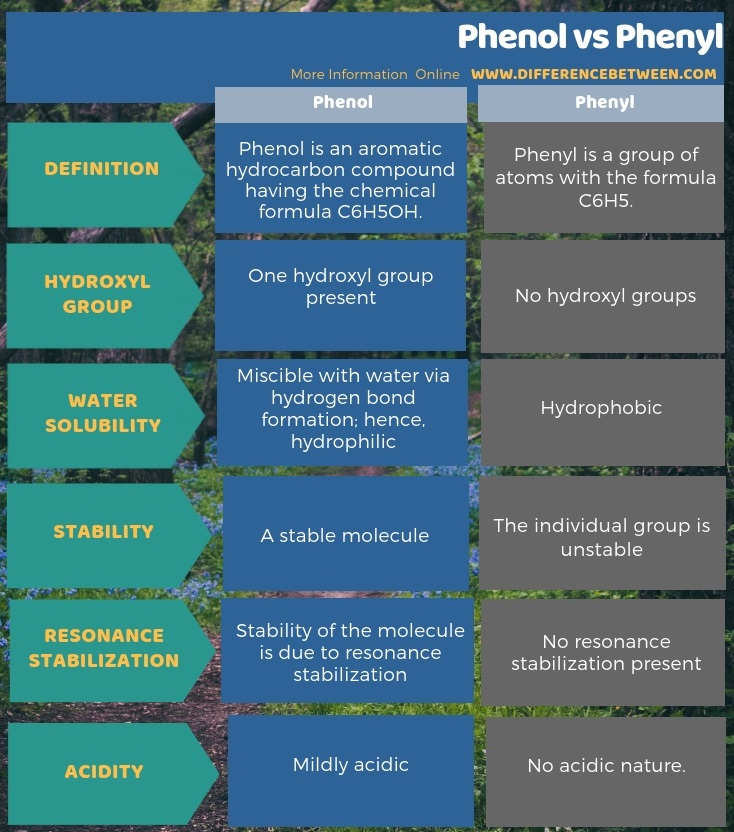
Phenol is an aromatic hydrocarbon compound having the chemical formula C6H5OH whereas phenyl is a group of atoms with the formula C6H5. Therefore, phenyl and phenol differ from each other due to the presence of a –OH group in phenol. Because of this, all the properties of the two differ. The key difference between phenol and phenyl is that phenol has a hydroxyl group (-OH) whereas phenyl has no hydroxyl group.
As another important difference between phenol and phenyl is that phenol is moderately soluble in water because it can form hydrogen bonds with water while Phenyl is hydrophobic. Moreover, Phenyl cannot be considered as a stable molecule itself, because it is a substituent. Phenol is actually a phenyl derivative with a –OH group. Also, one more difference between phenol and phenyl is that Phenyl cannot be resonance stabilized or don’t have acidic nature like phenol.
Summary – Phenol vs Phenyl
Phenyl is a group of atoms that derive from phenol via the removal of hydroxyl group (-OH). The key difference between phenol and phenyl is that phenol has a hydroxyl group whereas phenyl has no hydroxyl group.
Reference:
1. “Phenol.” Wikipedia, Wikimedia Foundation, 31 Oct. 2018. Available here
2. “Phenyl Group.” Wikipedia, Wikimedia Foundation, 15 Dec. 2017. Available here
Image Courtesy:
1.”Phenol-2D-skeletal”By Ben Mills – Own work, (Public Domain) via Commons Wikimedia
2.”Phenyl-group-2D-flat”By No machine-readable author provided (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau268x56lqKRdlruledWsZKmglaPGrXs%3D