Difference Between Nylon 6 and Nylon 66
Table of Contents
Key Difference – Nylon 6 vs Nylon 66
Nylon 6 and nylon 66 are the most frequently used nylon types in the world. The key difference between nylon 6 and nylon 66 is that nylon 6 is a monadic nylon derived from a diamine, while nylon 66 is a dyadic nylon derived from a diamine and a diacid.
Nylon refers to any polymer that comes under polyamides, which has amide linkages in their polymer backbone. There are various types of nylon fibers with a wide range of properties depending on their applications. In general, the most characteristic feature of nylon fiber is their strength and light weight. Moreover, they possess very high abrasion resistance, unlike many other synthetic fibers. Nylon is extremely elastic and they second only to spandex yarn and rubber. Nylon is resilient, making it resistant to wrinkles. The silky luster of nylon gives an appearance similar to cotton and wool. A tightly woven nylon fabric may feel light, but it traps moisture, air, and heat. Therefore, it is ideal to make fabrics of umbrellas and raincoats. Insects and mildew do not affect nylon. However, exposure to sunlight can reduce the properties of nylon. Some more drawbacks of nylon include the attraction of lint and dirt and static buildup. The major applications areas of nylon include consumer electronics, automotive industry, packaging etc. There are two types of nylons, namely; the monaidic (-[RNHCO]n-), and the dyadic (-[NHRNHCOR’COn]-). The type of nylon is often abbreviated as ‘nylon x’ or ‘nylon xy’, where x and y represent the number of carbon atoms in the monomer(s) from which they are synthesized.
CONTENTS
1. Overview and Key Difference
2. What is Nylon 6
3. What is Nylon 66
4. Side by Side Comparison – Nylon 6 vs Nylon 66 in Tabular Form
5. Summary
What is Nylon 6?
Nylon 6 is one of the most important monadic nylons mainly used as a fibre-forming polymer and as an engineering plastic. Nylon 6 is synthesized by melt polymerization of either Ɛ-aminocaproic acid or Ɛ- caprolactam. Nylon 6 absorbs moisture up to a certain extent, and the Tg (glass transition temperature) of the nylon 6 is reduced with increasing moisture content.
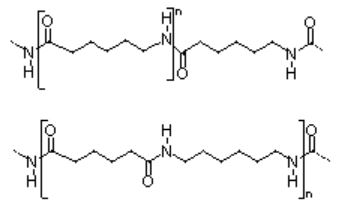
Figure 01: Nylon 6 and Nylon 66
The zig-zag molecular conformation and the anti-parallel arrangement of Nylon 6 chains result in more hydrogen bonds between amide groups. The tensile properties of Nylon 6 also reduce with increasing moisture content. The tenacity of fibres can be improved by melt spun and hot drawing of fibres. When compared with nylon 66, nylon 6 has high impact strength. Cast nylon 6 is used to manufacture hug gears and bearings, fuel tanks, building shutters, and various parts of paper production machinery and construction equipment. Fiberglass reinforced nylon 6 resins are used produce automotive radiator shrouds, air ducts, structural components, fuel cells, and reservoirs.
What is Nylon 66?
Nylon 66 is a dyadic nylon produced by high-temperature melt polymerization of adipic acid and hexamethylenediamine. Nylon 66 is one of the most important and highly used nylons in the world, owing to superior balance of properties and the relatively low price. The melting point of nylon 66 is around 260- 265 °C and Tg is about 50 °C when dry. Similar to nylon 6, nylon 66 consists of zig-zag chain conformation, resulting in intramolecular hydrogen bonds. Glass-fiber filled nylon 66 has excellent specific stiffness and toughness that enable it to be used in applications such as molded industrial drills and pump housing products. The tensile strength of nylon 66 is greater than that of nylon 6. The applications of molded nylon 66 include lawn mower blades, tractor hood extensions, bicycle wheels, skate wheels, skis for snowmobiles, bearings, electrical connections, and motorcycle crankcases. Nylon 66 fibers are used in clothing, fabric and rug industries.
What is the Difference Between Nylon 6 and Nylon 66?
Nylon 6 vs Nylon 66 | |
| Nylon 6 is a monadic nylon derived from Ɛ-aminocaproic acid or Ɛ- caprolactam | Nylon 66 is a dyadic nylon derived from adiapic acid and hexamethylenediamine |
| Chemical Name | |
| poly-(6-aminocarproic acid) | poly-[imino-(1,6-dioxohexamethylene)iminohexamethylene] |
| Chemical Formula | |
| -(-NH-(CH2)5-CO-)- | -(NH-(CH2)6-NH-CO-(CH2)4-CO-)- |
| Crystalline Melting Point | |
| Crystalline melting point is 225 °C. | Crystalline melting point is 265 °C. |
| Impact Strength (Izod: cm-N/cm of notch) | |
| 160 | 80 |
| Density | |
| 1.15 g/mL | 1.2 g/mL |
| Recyclability | |
| Nylon 6 can be recycled more times than nylon 66. | Nylon 66 is not as recyclable as nylon 6. |
| Tensile Strength | |
| 6.2 x 104 kPa | 8.3 x 104 kPa |
Summary – Nylon 6 vs Nylon 66
Nylon 6 and nylon 66 are among the most important and widely using polyamides. Nylon 6 is a monadic nylon derived from Ɛ-aminocaproic acid or Ɛ- caprolactam, whereas nylon 66 is a dyadic nylon derived from adipic acid and hexamethylenediamine. This is the main difference between Nylon 6 and Nylon 66. Both types have high stiffness and toughness, hence use as engineering plastics in many industrial applications.
Reference:
1. Chanda, Manas, and Roy Salil K. “Industrial Polymers, Specialty Polymers, and Their Applications.” CRC Press, 2008.
2. Ibeh, Christopher C. Thermoplastic Materials: Properties, Manufacturing Methods, and Applications. CRC Press, 2011.
3. M, Alger. Polymer Science Dictionary. Springer Science & Business Media, 1996.
4. Carraher, Charles E. Carraher’s Polymer Chemistry. CRC Press, 2016.
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2662KWmp2VmYq6vsIyvqmamqaG8r3mVb2SnsZyku26CjJqlnWWerrmwuoxvbWaZopp6tbTEZqSoq6Ris7Ox0K6cp6ycrnq2v8SdZKexnKS7bsDYqZysZZmjerW0xGauqKqcmXq1tMRmop6xXZm2p7LEq5ynm5Vir6bA1p6cp2WerrmwuoxvZJqmlGK7urjOp2Rvbl2ewG7Ax5qrZqapobyveZVmoKxlkWK6sLrAnaCcZZ6uubC6jg%3D%3D