Difference Between Noble Gas Configuration and Electron Configuration
Table of Contents
The key difference between noble gas configuration and electron configuration is that a noble gas configuration has only electron pairs whereas an electron configuration can have both paired and unpaired electrons.
The term electron configuration refers to the electron sequence or the order of the electrons present in an atom of a particular chemical element. The term noble gas electron configuration indicates that all atomic orbitals are completely filled with electrons.
CONTENTS
1. Overview and Key Difference
2. What is Noble Gas Configuration
3. What is Electron Configuration
4. Side by Side Comparison – Noble Gas Configuration vs Electron Configuration in Tabular Form
5. Summary
What is Noble Gas Configuration?
Noble gas configuration is the electron configuration of a noble gas atom. The noble gas atoms are the atoms of group 18 chemical elements in the periodic table. The group 18 chemical elements are known as noble gas elements due to two reasons; first, these chemical elements are mostly nonreactive because of their completed electron configurations, and the second reason is that these chemical elements occur in gaseous phase in nature.

Figure 01: Different Noble Gases
There are four major types of atomic orbitals in a chemical element; s orbital, p orbital, d orbital and f orbital. The s atomic orbital contains a maximum of two electrons, the p orbital can hold six electrons, d orbital can hold ten electrons, and the f orbital can hold 14 electrons. In group 18 chemical elements, we can observe s2p6 electron configuration; here, the s and p atomic orbitals are completely filled with electrons. Therefore, there are no unpaired electrons in these atoms.
What is Electron Configuration?
Electron configuration is the distribution of electrons of an atom in its atomic orbitals. This term describes each electron in the atom as moving independently in an orbital, in an average field created by all other orbitals.
The electron configuration of an atom can be expressed as the sequence of electrons present in that atom in the form of distribution throughout the atomic orbitals of that atom. Some chemical elements such as noble gas atoms have completed atomic orbitals, and there are no unpaired electrons; however, most of the chemical elements we know have unpaired electrons in their electron configuration. For example, the electron configuration of Neon atom, a noble gas atom, has the electron configuration 1s22s22p6.
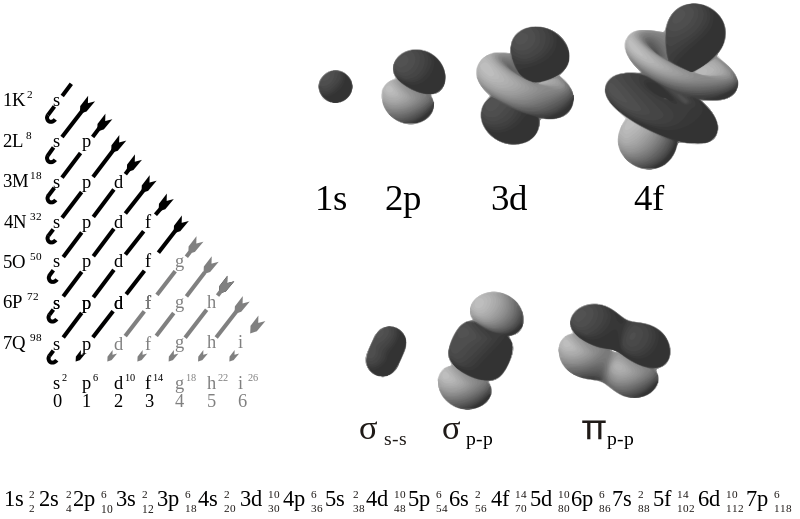
By looking at the electron configuration of an atom, we can describe the reactivity of that atom. A completely filled atomic orbital indicates an unreactive nature since it does not have to obtain any more electrons to stabilize itself. In contrast, in an atom having unpaired electrons often tend to be highly reactive in order to stabilize their electron configuration.
What is the Difference Between Noble Gas Configuration and Electron Configuration?
Noble gas configuration is the electron configuration of a noble gas atom; this means, the atom has completely filled atomic orbitals. The key difference between noble gas configuration and electron configuration is that noble gas configuration has only electron pairs whereas an electron configuration can have both paired and unpaired electrons. That means; the noble gas configuration has completely filled atomic orbitals while the electron configuration can have either completely filled or half-filled orbitals.
Below infographic lists more differences between noble gas configuration and electron configuration.
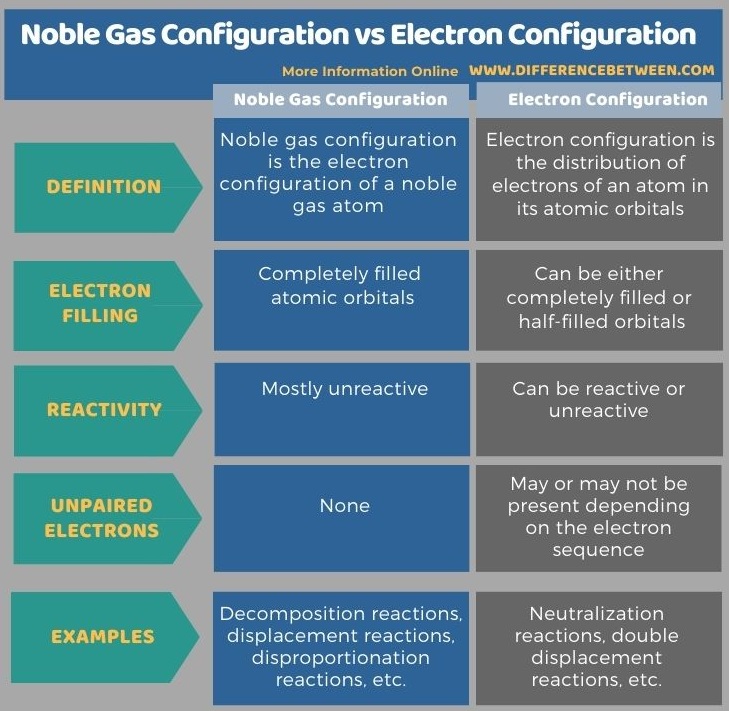
Summary – Noble Gas Configuration vs Electron Configuration
An electron configuration is the sequence of electrons that are present in an atom. The key difference between noble gas configuration and electron configuration is that noble gas configuration has only electron pairs whereas an electron configuration can have both paired and unpaired electrons.
Reference:
1. “Noble Gas.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 23 Oct. 2020, Available here.
Image Courtesy:
1. “Glowing noble gases” By New work Alchemist-hp (talk) www.pse-mendelejew.de); original single images: Jurii, http://images-of-elements.com. – Original: Jurii (CC BY 3.0) via Commons Wikimedia
2. “Electron orbitals” By Patricia.fidi – own work by Patricia.fidi and Lt Paul – Originally from pl:Grafika:Orbitale.png, author pl:Wikipedysta:Chemmix.This W3C-(Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau266zpujnmWXlsBur86nnaKfpaeutbXOp2SappRisq2xwq2pqKZdmLyvssigrKuZpJ68r3s%3D