Difference Between Methyl Acetate and Ethyl Acetate
Table of Contents
The key difference between methyl acetate and ethyl acetate is that methyl acetate has a methyl group attached to an acetate group whereas ethyl acetate has an ethyl group attached to an acetate group.
Acetate is an anion derived from the acetic acid (removal of the hydrogen atom in the carboxylic acid group forms the acetate anion). Both Methyl acetate and ethyl acetate are organic compounds with closely related chemical and physical properties.
CONTENTS
1. Overview and Key Difference
2. What is Methyl Acetate
3. What is Ethyl Acetate
4. Similarities Between Methyl Acetate and Ethyl Acetate
5. Side by Side Comparison – Methyl Acetate vs Ethyl Acetate in Tabular Form
6. Summary
What is Methyl Acetate?
Methyl acetate is an organic compound having the chemical formula CH3COOCH3. Here, the acetate (-COOCH3) group is attached to a methyl group (-CH3). The molar mass of the compound is 74 g/mol. It is categorized as a carboxylate ester since methyl acetate is formed by the interaction between carboxylate group and a methyl group, forming an ester bond.
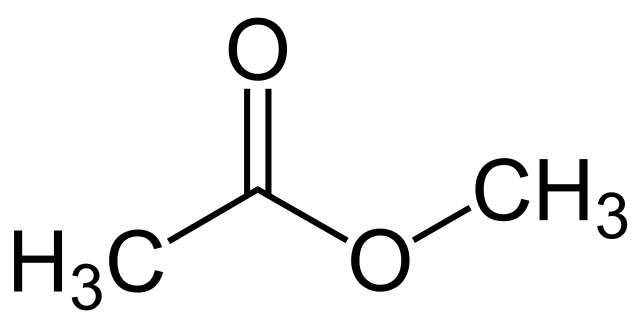
Figure 1: Methyl Acetate
At room temperature, methyl acetate is a colorless liquid with a fragrant odor. It also has a fruity taste. The melting point of this compound is -98°C while the boiling point is 56.9°C. This compound is moderately toxic. It is also a flammable liquid and has some uses as a solvent. Moreover, it is a weakly polar and lipophilic solvent. At room temperature, methyl acetate is poorly water soluble. But at higher temperatures, the compound has a high water solubility. Furthermore, Methyl acetate vapors are heavier than normal air.
What is Ethyl Acetate?
Ethyl acetate is an organic compound having the chemical formula CH3CH2COOCH3. The molar mass of this compound is 88 g/mol. It is categorized as a carboxylate ester since ethyl acetate is formed by the interaction between carboxylate group and an ethyl group, forming an ester bond. Furthermore, Ethyl acetate is the ester of ethanol and acetic acid.
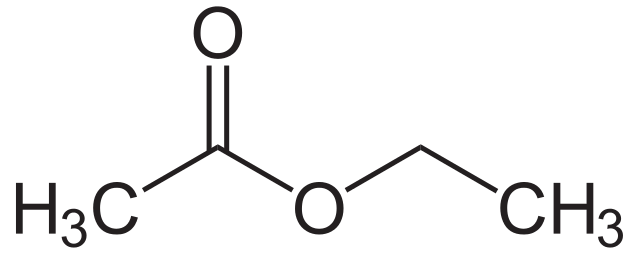
Figure 2: Ethyl Acetate
At room temperature, ethyl acetate is a colorless liquid with a fruity odor. This liquid is also widely used as a solvent. Ethyl acetate vapor is heavier than normal air. There a wide range of applications for this liquid because of its low cost, low toxicity, and pleasant odor.
The melting point of ethyl acetate is -83.6°C while the boiling point is 77°C. It is a flammable liquid and is an irritant. Moreover, the hydrolysis of Ethyl acetate results in acetic acid and ethanol. This hydrolysis is a two-step process that occurs in the presence of a strong base such as sodium hydroxide (NaOH). The first step involves the formation of ethanol and sodium acetate whereas the second step involves the conversion of sodium acetate into acetic acid.
What are the Similarities Between Methyl Acetate and Ethyl Acetate?
- Methyl Acetate and Ethyl Acetate are colorless liquids at room temperature with a fruity, pleasant odor.
- Both Methyl Acetate and Ethyl Acetate are flammable.
- Both compounds are carboxylate esters.
- Methyl Acetate and Ethyl Acetate are used as solvents.
What is the Difference Between Methyl Acetate and Ethyl Acetate?
Methyl Acetate vs Ethyl Acetate | |
| Methyl acetate is an organic compound having the chemical formula CH3COOCH3. | Ethyl acetate is an organic compound having the chemical formula CH3CH2COOCH3. |
| Molar Mass | |
| The molar mass of methyl acetate is 74 g/mol. | The molar mass of ethyl acetate is 88 g/mol. |
| Melting and Boiling Points | |
| The melting point of methyl acetate is -98°C while the boiling point is 56.9°C. | The melting point of ethyl acetate is -83.6°C while the boiling point is 77°C. |
| Toxicity | |
| Methyl acetate is moderately toxic. | Ethyl acetate is less toxic than Methyl acetate. |
| Use as a Solvent | |
| Methyl acetate is only occasionally used as a solvent. | Ethyl acetate is more widely used as a solvent. |
Summary – Methyl Acetate vs Ethyl Acetate
Both Methyl acetate and ethyl acetate are organic compounds with closely related chemical and physical properties. The key difference between methyl acetate and ethyl acetate is that methyl acetate has a methyl group attached to an acetate group whereas ethyl acetate has an ethyl group attached to an acetate group.
Reference:
1. “Methyl Acetate.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, Available here.
2. “Methyl Acetate.” Wikipedia, Wikimedia Foundation, 14 Apr. 2018, Available here.
3. “Ethyl Acetate.” Wikipedia, Wikimedia Foundation, 10 Apr. 2018, Available here.
Image Courtesy:
1. “Methyl-Acetate Structural Formula V1” By Jü – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Essigsäureethylester” By NEUROtiker (talk) – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau265xK2fsqRdlrCmwMCtnGaZnpl6psDHsqNmmZOawaLAxGg%3D