Difference Between Lactide and Lactone
Table of Contents
The key difference between lactide and lactone is that lactide is any heterocyclic compound formed by heating alpha-lactose whereas lactone is a cyclic ester that is derived from a hydroxy acid.
Lactide and lactone are two chemical terms that sound similar but have differences between them. Both these terms define subclasses of different chemical compounds that are cyclic and contain ester groups as their functional group.
CONTENTS
1. Overview and Key Difference
2. What is Lactide
3. What is Lactone
4. Side by Side Comparison – Lactide vs Lactone in Tabular Form
5. Summary
What is Lactide?
Lactide is a form of lactone that is derived from lactic acid upon heating. It is a cyclic diester compound. The chemical formula of lactide is C6H8O4 while the molar mass of this compound is 144 g/mol. When dissolved in water, lactide converts into lactic acid via hydrolysis reaction. Moreover, lactide is soluble in chloroform, methanol, benzene, etc. as well.
Furthermore, lactide shows stereoisomerism. There are three different stereoisomers of lactide. They are named R, R-lactide, S, S-lactide and meso-lactide isomer. Among them, R, R-isomer and S, S-isomer are enantiomers of each other, and they do not racemize readily. It is why lactide has three isomers, not two. Moreover, all three isomers of lactide undergo epimerization. This epimerization occurs in the presence of organic or inorganic bases. All three isomeric forms of lactide exist as white coloured solids.
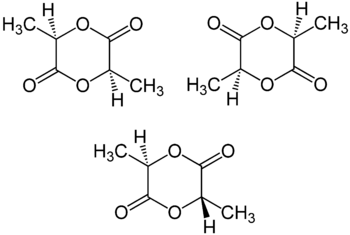
Figure 01: Chemical Structures of Three Isomers of Lactide
Lactide is useful as a precursor for some polymer materials such as polystyrene. However, it makes the polymer material biodegradable. In addition, lactide can be obtained from abundant renewable sources, which makes it an interest in research studies. Upon polymerization, lactide becomes polylactic acid. This product is also named polylactide. This polymerization reaction requires a catalyst, and depending on the type of catalyst, the reaction will give either syndiotactic or heterotactic polymers.
What is Lactone?
Lactones are a group of carboxylic esters that are cyclic and ketones. These compounds are formed from the esterification of hydroxycarboxylic acids (intermolecular esterification). This reaction is spontaneous when a five-membered or a six-membered ring forms. However, there are three-membered and four-membered rings in lactones as well. But they are very unreactive. This makes the isolation of these compounds very difficult. Therefore, these ring structures with a low number of carbon atoms in the ring need more Complicated laboratory methods for their separation.
Moreover, lactones have natural sources. For example, lactones can be found as building blocks of ascorbic acid, kavain, gluconolactone, some hormones, etc. Also, lactones can be synthesized in ester synthesis reactions.

Figure 02: Different Structures for Lactone Rings
Lactones are useful as flavouring agents and for fragrance. They are used as food additives to get the flavour of fruits and fermented dairy products. In addition, polymerization of lactones leads to the formation of the plastic “polycaprolactone”.
What is the Difference Between Lactide and Lactone?
The key difference between lactide and lactone is that a lactide is any of the class of heterocyclic compounds formed by heating alpha-lactose whereas lactone is a cyclic ester that is derived from a hydroxy acid.
The following infographic summarizes the difference between lactide and lactone.
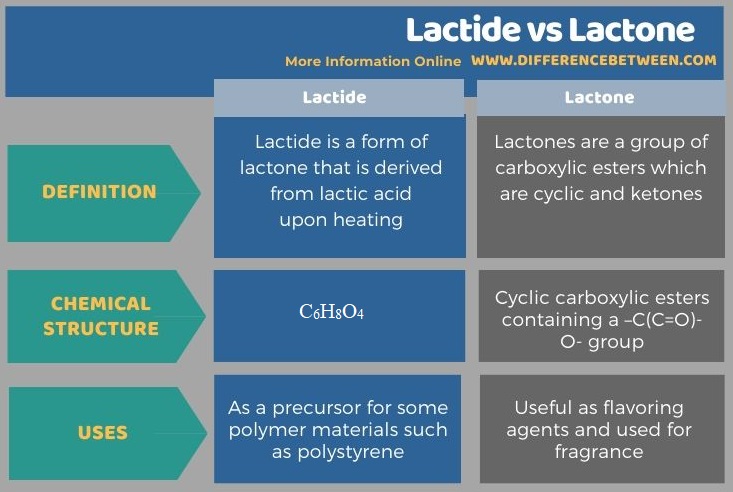
Summary – Lactide vs Lactone
Although the terms lactide and lactone sound the same, they are two different nouns. The key difference between lactide and lactone is that a lactide is any of the class of heterocyclic compounds formed by heating alpha-lactose whereas lactone is a cyclic ester that is derived from a hydroxy acid.
Reference:
1. “Lactide.” Wikipedia, Wikimedia Foundation, 7 Dec. 2019, Available here.
2. Jerome, Anrys. “Products.” Futerro, Available here.
3. “Lactone.” Wikipedia, Wikimedia Foundation, 12 Jan. 2020, Available here.
Image Courtesy:
1. “Lactide Stereoisomers Structural Formulae” By Jü – Jü (Public Domain) via Commons Wikimedia
2. “Lactone Types” By Cvf-ps – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau264wJyropyVYq6vsIylmJysn6OycA%3D%3D