Difference Between Isoelectric and Isoionic Point
Table of Contents
Key Difference – Isoelectric vs Isoionic Point
The two terms isoelectric point and Isoionic point describe the same biochemical concept about amino acids; the isoelectric point or Isoionic point is the pH at which the positive charges of an amino acid equals the negative charges of the same amino acid. Thus, there is no difference between the terms isoelectric point and Isoionic point.
CONTENTS
1. Overview and Key Difference
2. What is Isoelectric Point or Isoionic Point
3. Isoelectric vs Isoionic Point
4. Summary
What is Isoelectric Point or Isoionic Point?
Isoelectric point or Isoionic point of an amino acid is the pH at which the positive charges of an amino acid equals the negative charges of the same amino acid. It is denoted by pI. Since there is no net electrical charge in the amino acid, it cannot migrate in an electrical field. Therefore, isoelectric point is the point at which the amino acid is neutral.
At isoelectric point, a zwitterion is formed. A zwitterion is a dipolar molecule that has more than two functional groups having positive and negative charges (positively charged functional groups and negatively charged functional groups). The positive charges on functional groups should be equal to the negative charges present on functional groups of the amino acid. This makes the net electrical charge of the zwitterion zero.
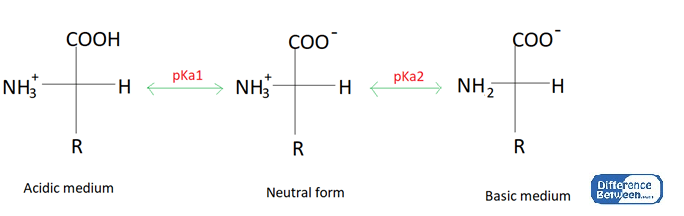
Figure 01: The acidic, basic and neutral forms of an Amino Acid
At the isoionic point, the zwitterion is the dominant form of the amino acid. The pI value can be obtained using the pKa values of net positive charge and net negative charge forms of the amino acids in acidic and basic mediums.
- If there are no charged functional groups in the side chain of the amino acid,
pI = ½(pKa1 + pKa2) where pKa1 and pKa2 are the pKa values of the amino acid in acidic and basic mediums.
- If there are acidic side chains, pI is lower than expected.
- If there are basic side chains, pI is higher than expected.
What is the Difference Between Isoelectric and Isoionic Point?
- Isoelectric point, also known as the Isoionic point, of an amino acid is the pH at which the positive charges of an amino acid equals the negative charges of the same amino acid, denoted by pI.
Summary – Isoelectric vs Isoionic Point
There is no difference between the terms isoelectric point and Isoionic point. Both terms are used to name the pH at which there is no net electrical charge in an amino acid.
Reference:
1. Hunt, Ian R. “Isoelectronic Point, PI.” Ch27: Isoelectronic Point. Available here
2. “Isoionic Point.” Wikipedia, Wikimedia Foundation, 17 Feb. 2018. Available here
3. “Zwitterion.” Wikipedia, Wikimedia Foundation, 20 Mar. 2018. Available here
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2610qicpZ2Tqb%2Bqr4yapZ1lpqh6qr%2FOoqanoZNivbC1za1m