Difference Between Ionomers and Polyelectrolytes
Table of Contents
The key difference between ionomers and polyelectrolytes is that ionomers are polymers containing both electrically neutral and ionized groups, whereas polyelectrolytes are polymers containing electrolytic groups.
Polymers are macromolecules composed of a large number of repeating units. These repeating units represent the monomers used in the formation of the polymer material. The process of formation of a polymer is called polymerization. Depending on the type of monomers used in polymerization, there are different types of polymers such as ionomers and polyelectrolytes.
CONTENTS
1. Overview and Key Difference
2. What are Ionomers
3. What are Polyelectrolytes
4. Side by Side Comparison – Ionomers vs Polyelectrolytes in Tabular Form
5. Summary
What are Ionomers?
Ionomers are polymer materials containing both neutral and ionized groups. These groups occur as pendent groups attached to the backbone of the polymer material via covalent bonding. Usually, an ionomer does not contain more than 15% ionized groups. Often, these ionized groups are carboxylic acid groups.
Since there are many different types of polymers, the type of pendent groups and the ways they are substituted to the polymer material should be considered in order to classify a particular material as an ionomer. For example, if the amount of ionized groups in the polymer exceeds 80%, then it is categorized as a polyelectrolyte, and if there are ionized groups attached as moieties of the backbone of the polymer, then they are classified as ionenes.
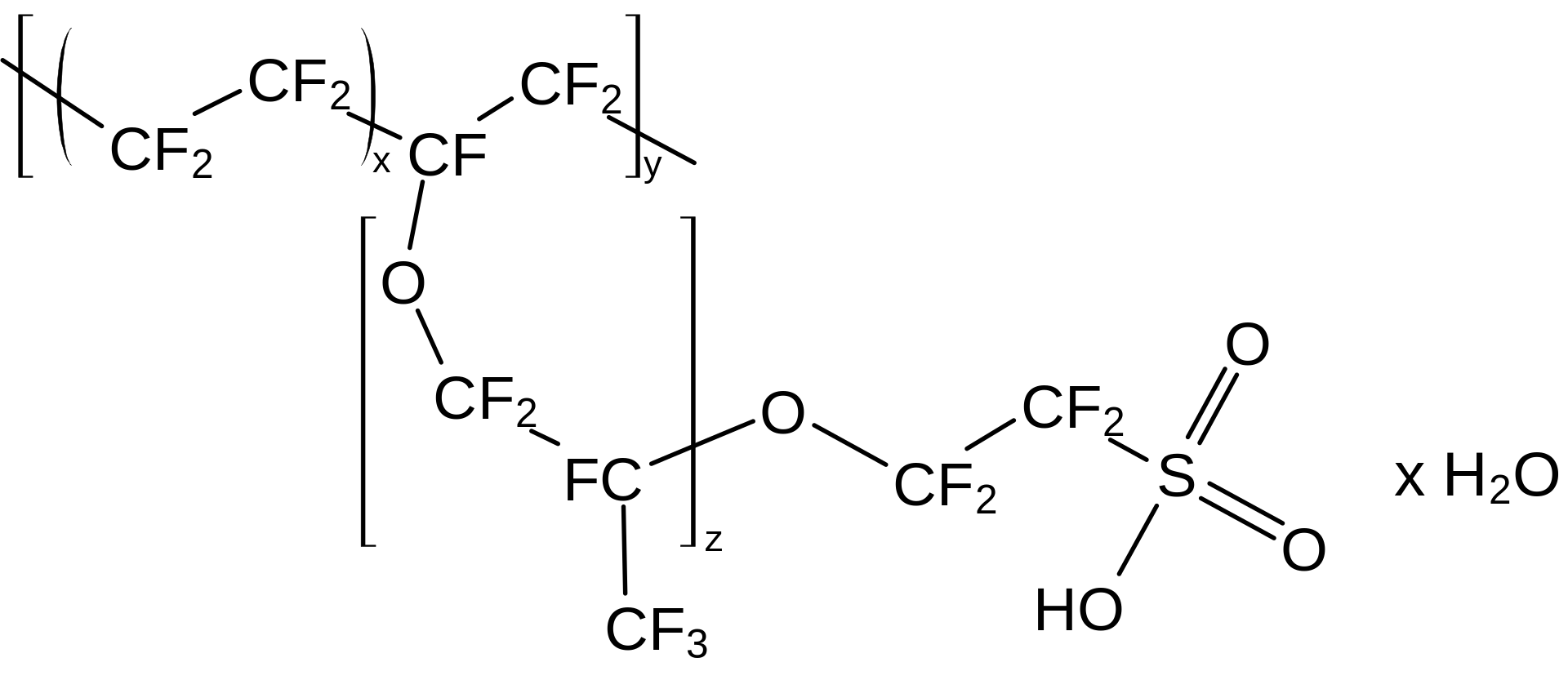
Figure 01: Nafion Polymer Structure – an Example for an Ionomer
Ionomers have unique properties, including electrical conductivity and viscosity. E.g. the viscosity of an ionomer solution increases with the temperature increment. Also, these materials have unique morphological properties: E.g. incompatible nonpolar backbone and polar ionic groups. Applications of ionomers include the production of golf bar covers, semipermeable membranes, sealing tapes, etc.
What are Polyelectrolytes?
Polyelectrolytes are polymer materials containing electrolytic groups. There are ionic pendant groups attached to the main backbone of the polymer material. According to the type of ionic group, there are two types as polycationic and polyanionic polymers. Usually, if the amount of ionized groups attached to the backbone exceeds 80%, then it is categorized as a polyelectrolytic polymer.
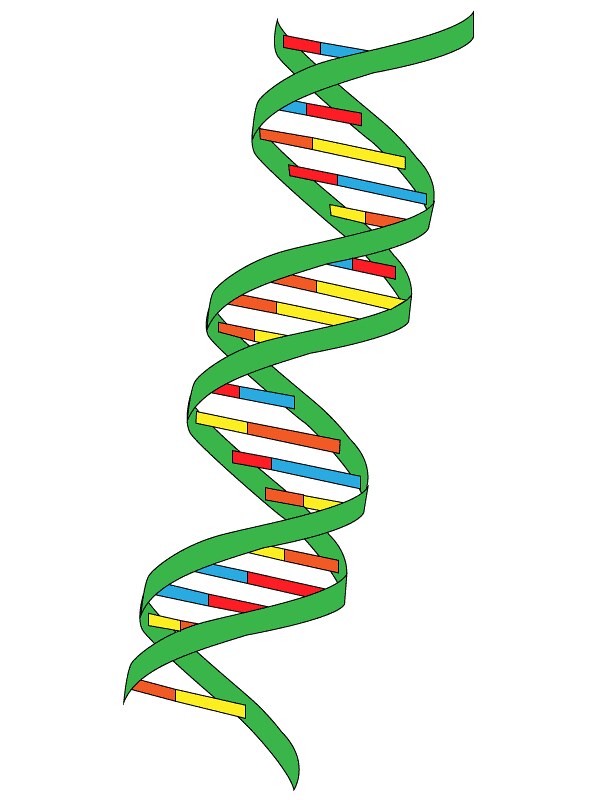
Figure 02: DNA is a Polyelectrolyte
When added to water, these polymer materials dissociate, making the polymer charged. Sometimes, these are called polysalts because their properties are similar to both salts and polymers. For example, aqueous solutions of polyelectrolytes are electrically conductive, similar to slats and the solutions are viscous, similar to polymers.
Some examples of polyelectrolytes include polypeptide, DNA, glycosaminoglycan, etc. There are many applications of these materials, including destabilization of colloidal suspension and initiation of flocculation, used to impart a surface charge to neutral particles, as thickeners, emulsifiers, conditioners, etc.
What is the Difference Between Ionomers and Polyelectrolytes?
The key difference between ionomers and polyelectrolytes is that ionomers are polymers containing both electrically neutral and ionized groups, whereas polyelectrolytes are polymers containing electrolytic groups. Moreover, ionomers do not contain more than 15% ionized groups, while polyelectrolytes contain more than 80% polyelectrolytes.
The following table summarizes the difference between ionomers and polyelectrolytes.
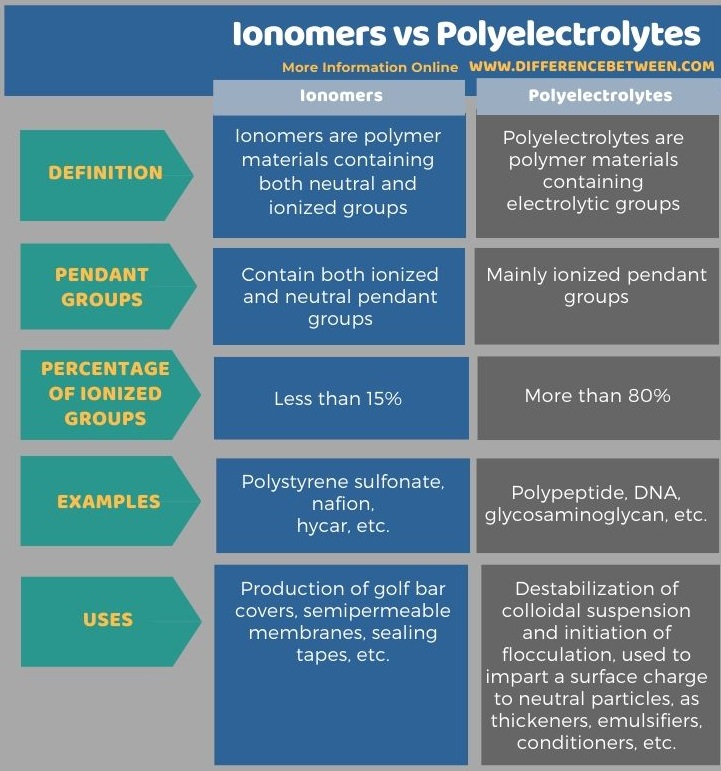
Summary – Ionomers vs Polyelectrolytes
Ionomers and polyelectrolytes are two types of polymer materials. These polymers are divided into groups according to the type of monomer used to form the polymer. The key difference between ionomers and polyelectrolytes is that ionomers are polymers containing both electrically neutral and ionized groups, whereas polyelectrolytes are polymers containing electrolytic groups.
Reference:
1. “Ionomers.” Ionomers – an Overview | ScienceDirect Topics, Available here.
2. “Polyelectrolyte.” Polyelectrolyte – an Overview | ScienceDirect Topics, Available here.
3. “Ionomer.” Wikipedia, Wikimedia Foundation, 5 May 2020, Available here.
Image Courtesy:
1. “Nafion2” By Roland1952 – Roland1952 (Public Domain) via Commons Wikimedia
2. “vector DNA color” By Greg Emmerich (CC BY-SA 2.0) via Flickr
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau261zqempp2iqHqiusNmp6ikqZq5pq%2FTq6alsaSawHA%3D