Difference Between Iodine and Potassium Iodide
Table of Contents
The key difference between iodine and potassium iodide is that iodine is a chemical element whereas potassium iodide is a chemical compound.
Iodine is a halogen that locates in group 17 of the periodic table of elements. On the other hand, potassium iodide is a chemical compound that forms from the combination of iodine and potassium. As a result, potassium iodide is very useful in many industries as a source of iodine.
CONTENTS
1. Overview and Key Difference
2. What is Iodine
3. What is Potassium Iodide
4. Side by Side Comparison – Iodine vs Potassium Iodide in Tabular Form
5. Summary
What is Iodine?
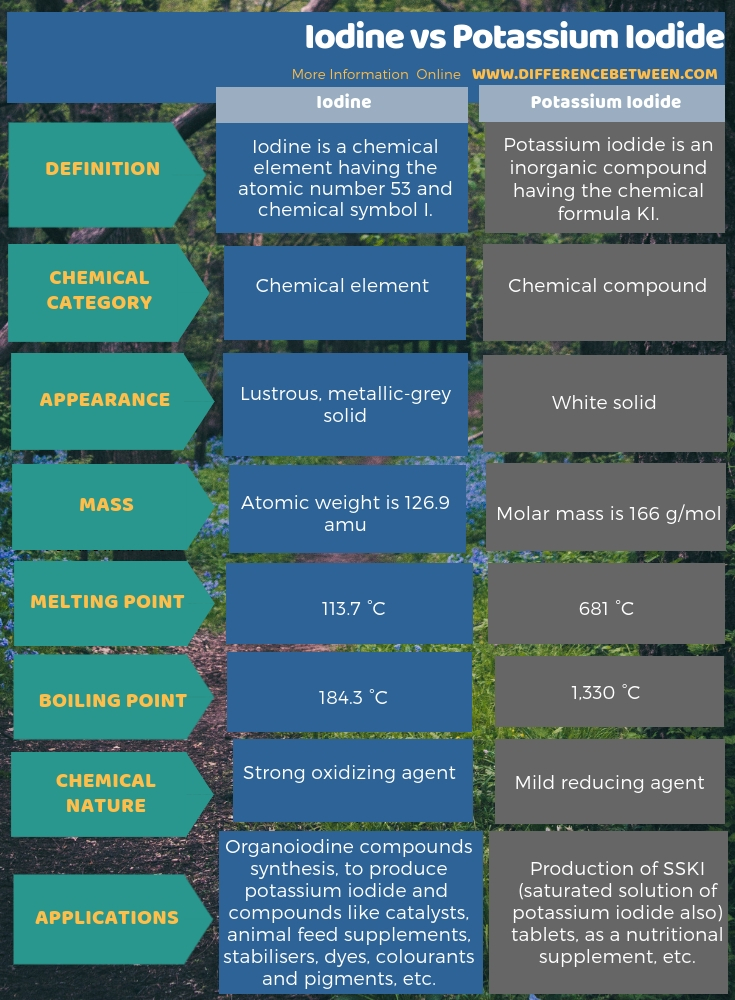
Iodine is a chemical element having the atomic number 53 and chemical symbol I. It is the heaviest halogen among the other halogens. The halogens are group 17 chemical elements in the periodic table. Furthermore, iodine exists as a lustrous, metallic-grey solid at room temperature. However, it can readily undergo sublimation to form the violet gas of iodine. Moreover, among the oxidation states that iodine can exist, the -1 oxidation is the most common among them, which results in the iodide anion. It is because, the iodine has an incomplete octet in its electron configuration in which it requires an electron to complete the octet. Then, when it obtains an electron from outside, it forms the -1 oxidation state.
Some important facts about iodine are as follows:
- Atomic number – 53
- Standard atomic weight – 126.9
- Appearance – lustrous, metallic-grey solid
- Electron configuration – [Kr] 4d10 5s2 5p5
- Group – 17
- Period – 5
- Chemical category – nonmetal
- Melting point is 113.7 °C
- Boiling point is 184.3 °C

Figure 01: A sample of Solid Iodine
Moreover, iodine is a strong oxidizer. Mainly, it is due to its incomplete octet of electron configuration in which it lacks one electron to fill up the outermost p orbital. Thus, it seeks for an electron by oxidizing other chemical species. However, it is the weakest oxidizing agent among other halogens due to its large atomic size.
What is Potassium Iodide?
Potassium iodide is an inorganic compound and appears as white solid and is produced commercially in large quantities. It is the most important iodide compound because it is less hygroscopic than any other iodide compounds. The chemical formula of this compound is KI.
Some important chemical facts about this compound are as follows:
- Chemical formula – KI
- Molar mass – 166 g/mol.
- Melting point is 681 °C.
- Boiling point is 1,330 °C.
- It has the crystal structure of sodium chloride.
- A mild reducing agent.
- Production is industrially by treating KOH with iodine.

Figure 02: A sample of Solid Potassium Iodide
The most important application of KI is in the form of SSKI (saturated solution of potassium iodide also) tablets. These tablets are taken in the emergency treatment of several ailments. Also, SSKI is useful for the treatment in the cases of exposure to nuclear accidents. Furthermore, KI is a supplement for iodine deficiency when added to table salt. Moreover, we can use it in the photography industry and in the field of biomedical research.
What is the Difference Between Iodine and Potassium Iodide?
Iodine is a chemical element having the atomic number 53 and chemical symbol I whereas Potassium iodide is an inorganic compound and appears as white solid and is produced commercially in large quantities. Therefore, the key difference between iodine and potassium iodide is that iodine is a chemical element whereas potassium iodide is a chemical compound. In brief, iodine combines with potassium (e.g., KOH) to produce potassium iodide compound. As another important difference between iodine and potassium iodide, we can say that iodine has a lustrous, metallic-grey appearance whereas potassium iodide appears as a white solid compound.
Furthermore, there is a difference between iodine and potassium iodide in their usage as well. Also, there are few other differences in their chemical properties too. The below infographic summarizes the difference between iodine and potassium iodide in tabular form.
Summary – Iodine vs Potassium Iodide
Iodine being a halogen cannot remain as an element under standard temperature and pressure but combines with other elements to form compounds easily. Hence, it is this property to form compounds that make it a very important element. Therefore, the key difference between iodine and potassium iodide is that iodine is a chemical element whereas potassium iodide is a chemical compound. Iodine combines with potassium to form potassium Iodide which is a very important compound that is commercially useful in various industries. However, iodine isotopes are dangerous for humans, but when this iodine is taken in the form of KI, it becomes useful to humans. Moreover, iodine deficiency leads to mental retardation and goitre, this deficiency is fulfilled by the administration of iodine in the form of KI.
Reference:
1.“Iodine.” Wikipedia, Wikimedia Foundation, 20 Oct. 2018. Available here
2.“Potassium Iodide.” Wikipedia, Wikimedia Foundation, 26 Sept. 2018. Available here
Image Courtesy:
1.”Sample of iodine”By LHcheM – Own work, (CC BY-SA 3.0) via Commons Wikimedia
2.”Potassium iodide” (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau261zp2gp51dlruledWsZKmnpJbAtLXUpmSip5SesaZ7