Difference Between Hypotonic and Hypertonic
Table of Contents
The key difference between hypotonic and hypertonic is that hypotonic solution has a low solute concentration than the cell while hypertonic solution has a high solute concentration than the cell.
Osmosis is the process of moving water molecules from high water potential to low water potential through a semi-permeable membrane. However, this semi-permeable membrane only allows solvent particles (water molecules) to move across it and does not allow solute particles to move through the membrane. Tonicity is a measure of the osmotic pressure gradient and there are three states of it. These are hypertonic, isotonic and hypotonic. Among the three solutions, hypotonic solution is the solution which has a low solute concentration while hypertonic solution is the solution which has a high solute concentration. The solvent concentration gradient across the two solutions is the driving force for this process. The net movement of the solvent from hypotonic solvent to hypertonic solvent takes place due to the unequal osmotic pressure.
CONTENTS
1. Overview and Key Difference
2. What is Hypotonic
3. What is Hypertonic
4. Similarities Between Hypotonic and Hypertonic
5. Side by Side Comparison – Hypotonic vs Hypertonic in Tabular Form
6. Summary
What is Hypotonic?
A hypotonic solution is a solution which has a less solute concentration compared to the inside of the cell. Hence, the osmotic pressure of this solution is very low compared to other solutions. When a cell is immersed in a hypotonic solution, water molecules move inside the cell from the solution due to the osmotic potential.
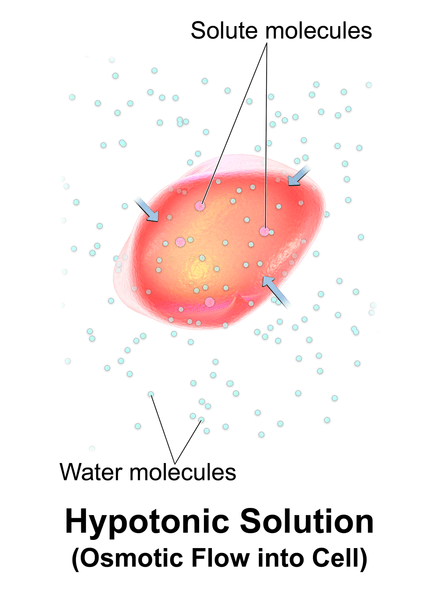
Figure 01: Hypotonic Solution
The continuous diffusion of water molecules into the cell would cause cell swelling. And, it may result in cytolysis of the cell (rupture). However, plant cells do not burst since they have a rigid cell wall.
What is Hypertonic?
A hypertonic solution has a high concentration of solutes than that of the inside of the cell. When a cell is immersed in a hypertonic solution, the water molecules come out from the cell to the solution. Due to the water movement from the cell to the outside, the cell becomes distorted and wrinkled. Thus, this effect is called ‘crenation’ of the cell.

Figure 02: Hypertonic Solution
In the plant cells, the flexible plasma membrane pulls away from the rigid cell wall, but remains joined to the cell wall at certain points due to the effect of crenation and finally results in a condition called ‘plasmolysis’.
What are the Similarities Between Hypotonic and Hypertonic?
- Hypotonic and hypertonic are two types of extracellular fluids that are described in terms of osmolarity.
- Both solutions have solvent molecules and solute molecules.
- In both solutions, there is a net movement of solvent molecules.
What is the Difference Between Hypotonic and Hypertonic?
A hypotonic solution is a solution that contains low solute concentrations while a hypertonic solution is a solution that contains high solute concentrations. So, this is the key difference between hypotonic and hypertonic. Besides, a hypotonic solution has a high water potential while a hypertonic solution has a low water potential. Therefore, this is also a significant difference between hypotonic and hypertonic solutions.
Moreover, a further difference between hypotonic and hypertonic solutions is that the water molecules move from a hypotonic solution to the cell while water molecules move from the cell to the hypertonic solution. Additionally, the cells shrink when placed in a hypertonic solution while the cells swell when placed in a hypotonic solution. Therefore, this is also an important difference between hypotonic and hypertonic.
The below info-graphic presents more information on the difference between hypotonic and hypertonic solutions, comparatively.
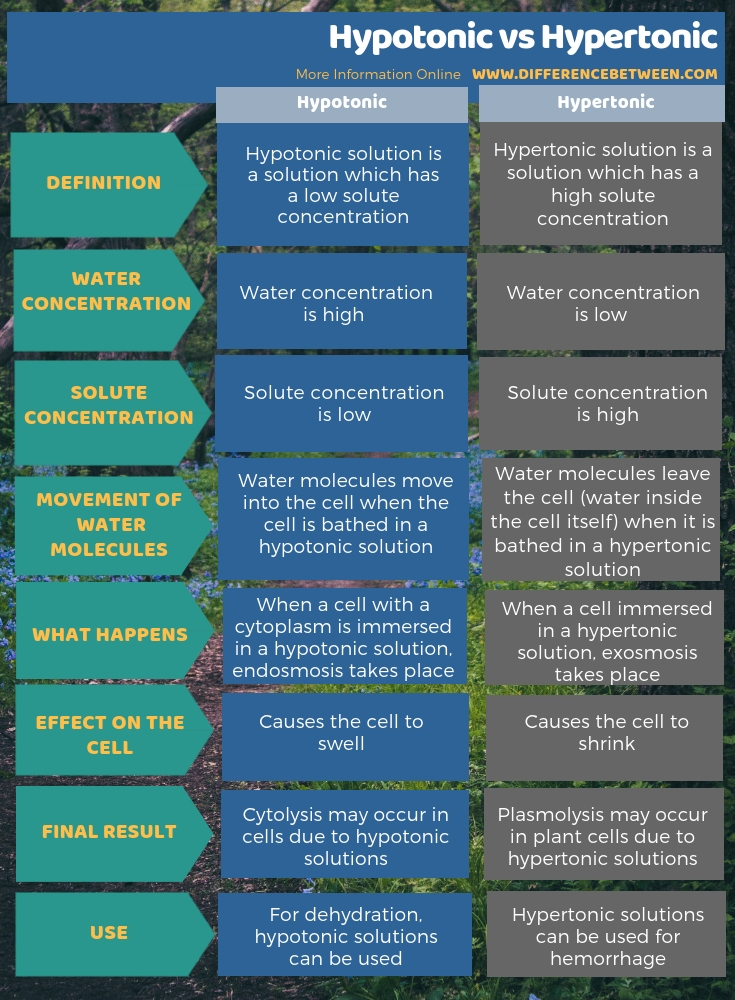
Summary – Hypotonic vs Hypertonic
Hypotonic and hypertonic are two types of solutions based on osmolarity. A hypotonic solution has a low solute concentration compared to the cell inside. Hence, water molecules move from the hypotonic solution to the cell. Due to the water movement into the cells, cells swell. On the other hand, a hypertonic solution has a high solute concentration compared to the cell. Hence, water molecules move from the cell to the solution. As a result, cells tend to shrink. Thus, this is a summary of the difference between hypotonic and hypertonic.
Reference:
1. “Osmosis and Tonicity.” Khan Academy, Khan Academy, Available here.
Image Courtesy:
1. “Blausen 0684 OsmoticFlow Hypotonic” By Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. – Own work (CC BY 3.0) via Commons Wikimedia
2. “Blausen 0683 OsmoticFlow Hypertonic” By BruceBlaus. When using this image in external sources it can be cited as:Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. – Own work (CC BY 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2602KmmraeenrBurc2dZK%2BrXZ3GsbHRraanoZNk