Difference Between Hydride and Methyl Shift
Table of Contents
The key difference between hydride and methyl shift is that a hydride shift can occur when a hydrogen atom moves to a carbon atom bearing a positive charge from an adjacent carbon in the same molecule, whereas methyl shift occurs when a methyl group moves to a carbon atom bearing a positive charge from an adjacent carbon atom in the same molecule.
The terms hydride shift and methyl shift come under the subtopic of carbocation rearrangements. Here, either a hydrogen atom or a methyl group moves to a charged carbon atom from an adjacent carbon atom in the same compound.
CONTENTS
1. Overview and Key Difference
2. What is Hydride Shift
3. What is Methyl Shift
4. Similarities between Hydride and Methyl Shift
5. Side by Side Comparison – Hydride vs Methyl Shift in Tabular Form
6. Summary
What is Hydride Shift?
Hydride shift is the movement of a hydrogen atom from one carbon to a charged, adjacent carbon atom of the same compound. Most frequently, the carbocation rearrangements occur in secondary carbocations. The rearranged carbocation is the major product of a synthesis reaction because it is the most stable form.
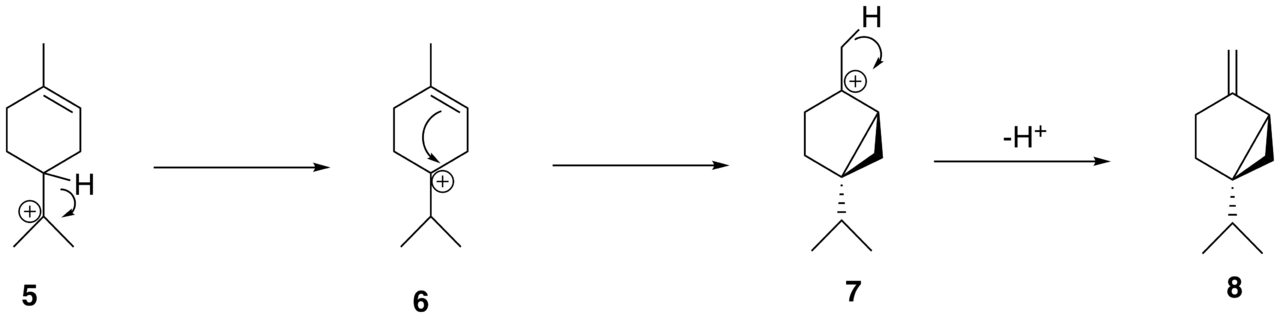
If a secondary carbocation is vicinal to a tertiary carbon atom bearing a hydrogen atom, then a hydride shift occurs. We call this a 1,2-hydride shift. This shift is possible when there is a positive charge on the carbon atom where its adjacent carbon atom has a removable hydrogen atom.
What is Methyl Shift?
Methyl shift is the movement of a methyl group from one carbon atom to a charged, adjacent carbon atom of the same compound. We call this a methyl shift if the moving chemical species is a methyl group, and it can be any other possible alkyl group as well. Here, the smaller substituent alkyl group tends to be the moving chemical species that attach to the charged carbon atom. The shifting of the methyl group is named as 1,2-methyl shift.
What are the Similarities between Hydride and Methyl Shift?
Generally, the mechanism for hydride shift and methyl shift are the same. Here, the arrow starts from the carbon atom where the substituent (hydrogen atom of the methyl group) attached to and points at the charged carbon atom. It shows that the hydrogen atom or the methyl group is migrating to the carbocation with its electrons. Both these shifts result in a new carbocation at the end of the rearrangement process. Moreover, the hydride shift or the methyl shift cannot be done for 1,3 or 1,4 shifts.
What is the Difference Between Hydride and Methyl Shift?
Hydride shift and methyl shift are types of carbocation rearrangements. The key difference between hydride and methyl shift is that a hydride shift can occur when a hydrogen atom moves to a carbon atom bearing a positive charge from an adjacent carbon in the same molecule, whereas methyl shift occurs when a methyl group moves to a carbon atom bearing a positive charge from an adjacent carbon atom in the same molecule. Besides, while the hydride shift is named as 1,2-hydride shift, the methyl shift is named as 1,2-methyl shift.
Below infographic summarizes the difference between hydride and methyl shift.
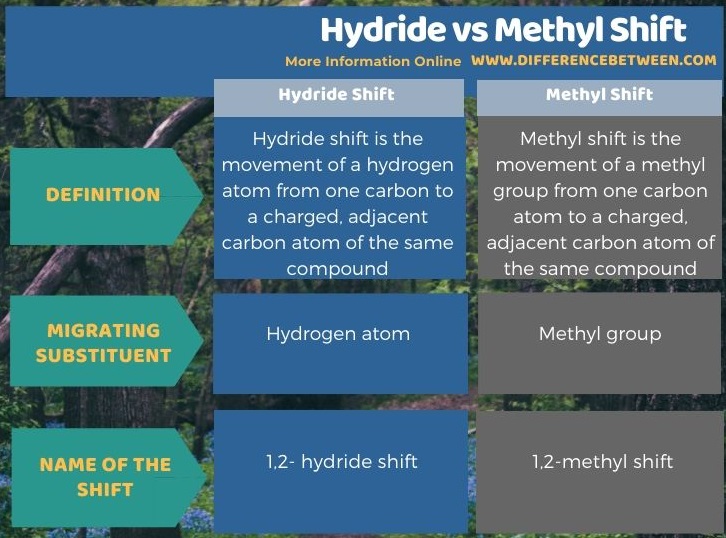
Summary – Hydride vs Methyl Shift
Hydride shift and methyl shift are types of carbocation rearrangements. The key difference between hydride and methyl shift is that a hydride shift can occur when a hydrogen atom moves to a carbon atom bearing a positive charge from an adjacent carbon in the same molecule whereas methyl shift occurs when a methyl group moves to a carbon atom bearing a positive charge from an adjacent carbon atom in the same molecule.
Reference:
1. “Carbocation Rearrangements.” Chemistry LibreTexts, Libretexts, 5 June 2019, Available here.
2. “1,2-Hydride Shift.” Chemistry LibreTexts, Libretexts, 5 June 2019, Available here.
Image Courtesy:
1. “The-conversion-of-alpha-terpinyl-cation-to-sabinene” By A1abbas – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2602J2popyVYq6vsIymnK2gqaF6tLTIn6to