Difference Between Homolytic and Heterolytic Fission
Table of Contents
The key difference between homolytic and heterolytic fission is that the homolytic fission gives one bond electron to each fragment whereas the heterolytic fission gives two bond electrons to one fragment and none of the bond electrons to the other fragment.
Fission is the destruction of a covalent chemical bond. In other words, it divides one molecule into two moieties. Fission comes in two forms as homolytic fission that forms two equal moieties and heterolytic fission that forms two unequal moieties.
CONTENTS
1. Overview and Key Difference
2. What is Homolytic Fission
3. What is Heterolytic Fission
4. Side by Side Comparison – Homolytic vs Heterolytic Fission in Tabular Form
5. Summary
What is Homolytic Fission?
Homolytic fission is the dissociation of a chemical bond and forming two equal fragments. A chemical bond (covalent bond) contains two electrons. In this form of fission, each of the fragments gets one unpaired electron. When this bond dissociation occurs in a neutral molecule that has an even number of electrons, it forms two equal, free radicals.
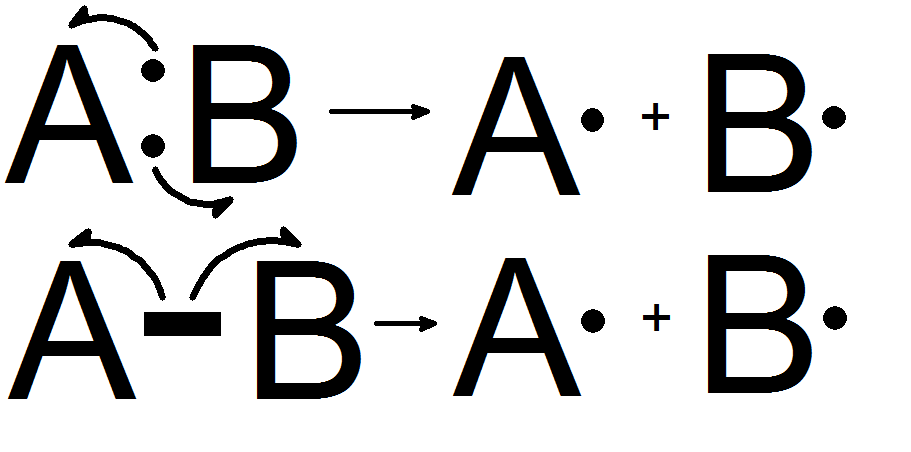
Figure 01: Homolytic Fission
Homolytic bond dissociation energy refers to the energy that is either absorbed or released during this process. However, this fission takes place only under specific conditions;
- UV radiation
- Heat
In addition to that, some certain chemical bonds such as peroxide bond are weak enough to dissociate spontaneously upon a slight heat.
What is Heterolytic Fission?
Heterolytic fission is the dissociation of a chemical bond and forming two unequal fragments. A chemical bond (covalent bond) contains two electrons. In this form of fission, one fragment gets both bond electron pairs while other fragment gets none of the bond electrons.

Figure 02: Heterolytic Fission
The fragment that gets both bond electrons form an anion. The other fragment forms a cation. There, the fragment which gets both electrons is more electronegative than the other fragment. This fission occurs in single covalent bonds. The energy that is either absorbed or released during this bond cleavage is called “heterolytic bond dissociation energy”.
What is the Difference Between Homolytic and Heterolytic Fission?
Homolytic fission is the dissociation of a chemical bond and forming two equal fragments. It gives one bond electron to each fragment. The energy that is either absorbed or released during homolytic fission is called the “homolytic bond dissociation energy”. Heterolytic fission is the dissociation of a chemical bond and forming two unequal fragments. It gives two bond electrons to one fragment and none of the bond electrons to the other fragment. This is the key difference between homolytic and heterolytic fission. The energy that is either absorbed or released during heterolytic fission is called the “heterolytic bond dissociation energy”.

Summary – Homolytic vs Heterolytic Fission
Fission is bond dissociation. It is in two forms as homolytic and heterolytic fission. The difference between homolytic and heterolytic fission is that the homolytic fission gives one bond electron to each fragment whereas the heterolytic fission gives two bond electrons to one fragment and none of the bond electrons to the other fragment.
Reference:
1. “Homolysis (Chemistry).” Wikipedia, Wikimedia Foundation, 17 July 2018. Available here
2. “Enzyme Activator.” Wikipedia, Wikimedia Foundation, 15 July 2018. Available here
Image Courtesy:
1.’Arrow Pushing Homolytic’By Shoy – Own work, (Public Domain) via Commons Wikimedia
2.’Heterolysis (Chemistry)’By Jürgen Martens – Jürgen Martens, (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau260zqampbGknrBurc2dZKGdpJq%2FsLjYraCcZZaewLS1zqdm