Difference Between Group 1 and Group 2 Elements
Table of Contents
The key difference between group 1 and group 2 elements is that all group 1 elements have unpaired electrons in their outermost orbital, whereas group 2 elements have paired electrons in their outermost orbital.
Groups 1 and 2 of the periodic table contain s block elements. That means; these elements have their outermost electrons in the s orbital. The group 1 and 2 differs from each other depending on the number of electrons in their outermost orbital. One s orbital can contain only two electrons because the magnetic quantum number of this orbital is 0.
CONTENTS
1. Overview and Key Difference
2. What are Group1 Elements
3. What are Group 2 Elements
4. Side by Side Comparison – Group1 vs Group 2 Elements in Tabular Form
5. Summary
What are Group 1 Elements?
Group 1 elements are chemical elements having an unpaired electron in the outermost s orbital. It is the first column of the s block of the periodic table. It contains hydrogen and alkali metals. The members of this group 1 are as follows:
- Hydrogen (H)
- Lithium (Li)
- Sodium (Na)
- Potassium (K)
- Rhubidium (Rh)
- Caesium (Cs)
- Francium (Fr)
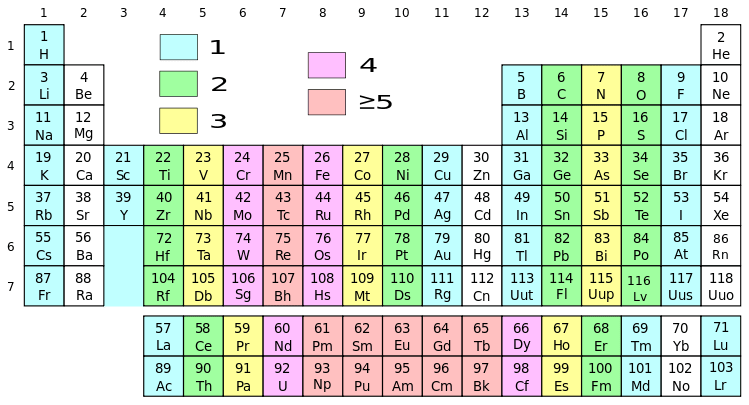
Figure 01: Periodic Table with Different Groups in Different Colors
Although hydrogen is in this group due to its electron configuration, it has characteristics distinct from alkali metals. For instance, hydrogen exists as a gas, while other elements in this group are metals. These metals are all shiny, highly reactive, and very soft (we can easily cut them using a simple knife).
Generally, the group 1 elements show low densities, low melting points, low boiling points and have body-centred cubic crystal structures. Moreover, they have distinct flame colours, so we can easily distinguish them by exposing a sample to a Bunsen burner. When going down the group of alkali metals, there are some periodic variations as listed below.
- The atomic size increases
- The melting point and boiling point decrease because of the ability to form strong bonds is decreased down the group (when the atom get large, the formed bond is weak).
- The density increases.
- First ionization energy decreases because in large atoms, the outermost electron is loosely bound and it can easily be removed.
- Electronegativity
- Reactivity decreases.
- Alkali metals have low electron affinities than other elements.
What are Group 2 Elements?
Group 2 elements are chemical elements having their outermost electron pair in an s orbital. Therefore, their valence electrons are in the form of ns2. Further, this group is the second column of the s block. We name them as alkaline earth metals. The members of this group are as follows:
- Beryllium (Be)
- Magnesium (Mg)
- Calcium (Ca)
- Strontium (Sr)
- Barium (Ba)
- Radium (Ra)
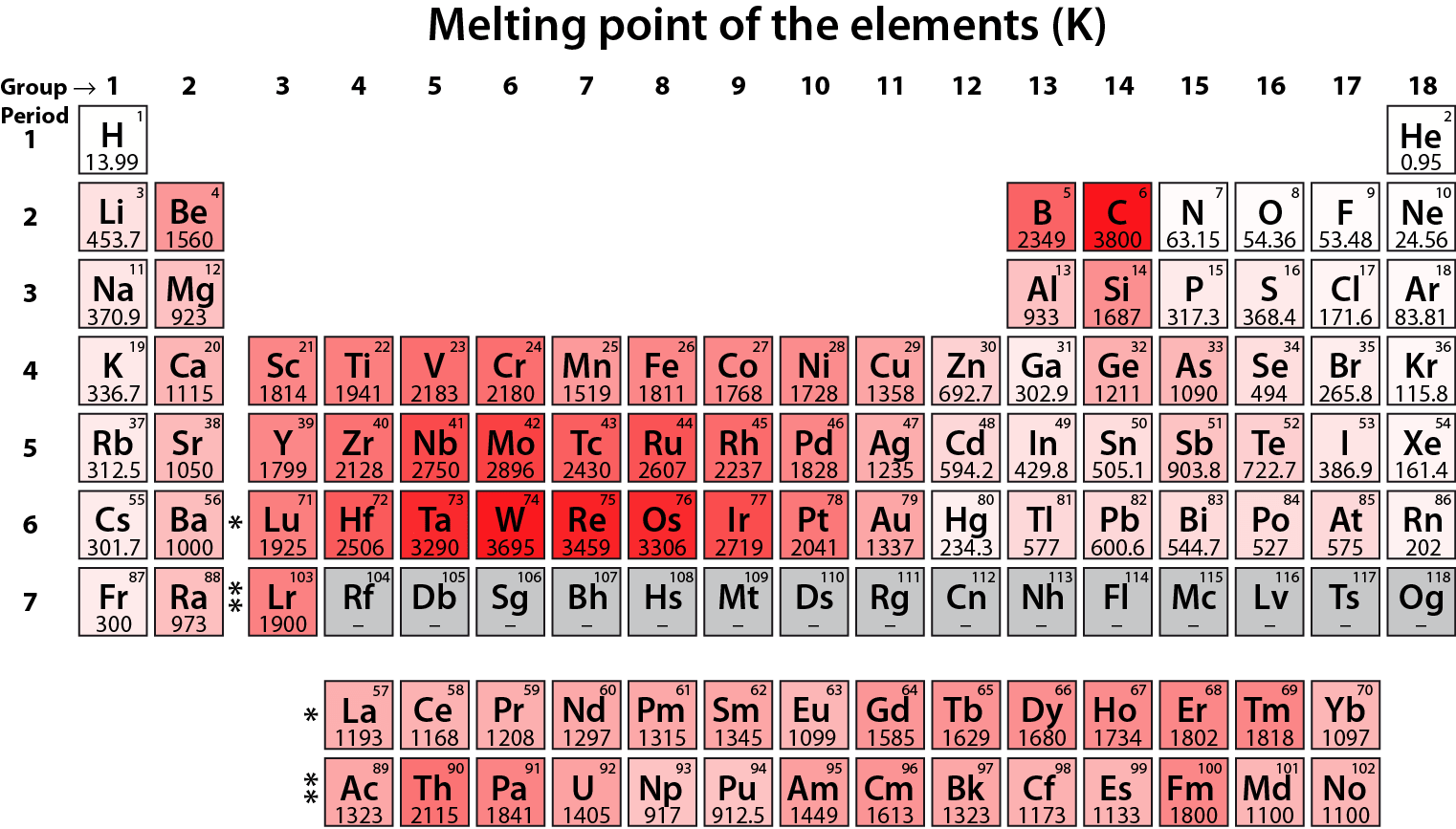
Figure 02: Melting Points of Elements
These metal elements tend to stabilize their electron configuration by removing two outermost s electrons to obtain a noble gas electron configuration. Therefore, these elements tend to form +2 cations. These metals are less reactive compared to group 1 elements. Moreover, these elements have higher melting points compared to group 1 elements, and their hydroxides are comparatively less basic.
What is the Difference Between Group 1 and Group 2 Elements?
The group 1 and 2 differ from each other depending on the number of electrons in their outermost orbital. The key difference between group 1 and group 2 elements is that all group 1 elements have unpaired electrons in their outermost orbital, whereas group 2 elements have paired electrons in their outermost orbital.
The below infographic shows more comparisons regarding the difference between group 1 and group 2 elements.
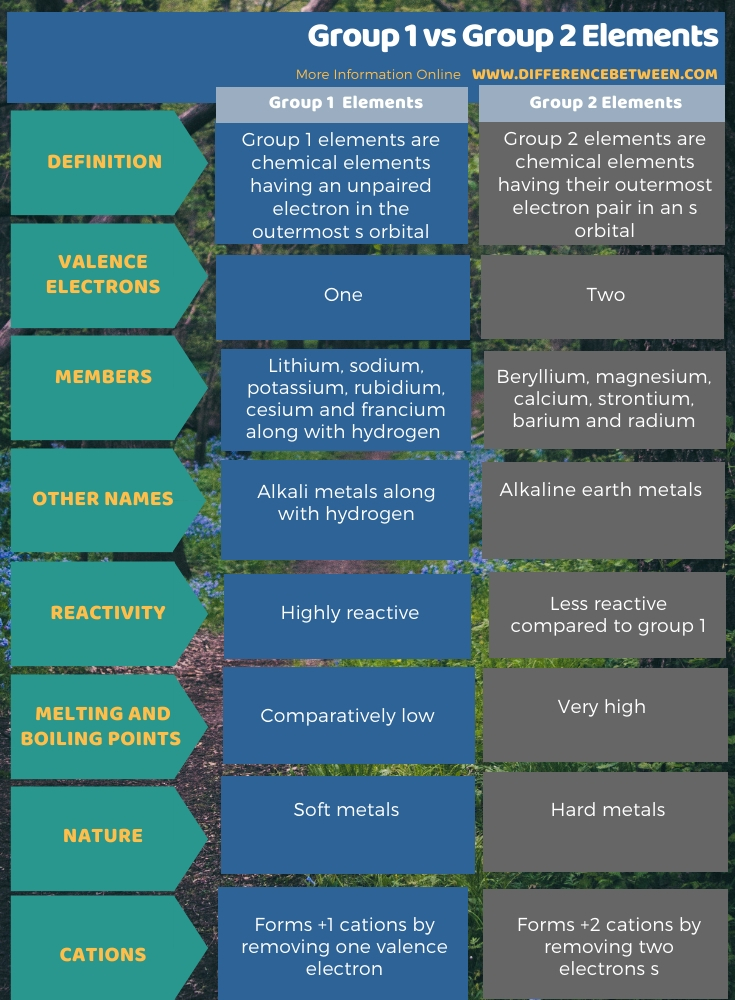
Summary – Group 1 vs Group 2 Elements
The group 1 and 2 differ from each other depending on the number of electrons in their outermost orbital. The key difference between group 1 and group 2 elements is that all group 1 elements have unpaired electrons in their outermost orbital, whereas group 2 elements have paired electrons in their outermost orbital.
Reference:
1.“Group 1: Hydrogen and the Alkali Metals.” Chemistry LibreTexts, Libretexts, 23 June 2019, Available here.
2. Helmenstine, Anne Marie. “Periodic Table of Element Groups.” ThoughtCo, Nov. 11, 2019, Available here.
Image Courtesy:
1. “Periodic Table with unpaired electrons” By KES47 – SVG version from an jpg image created by Sai2020 on 2009-02-03:File:Periodic Table with unpaired e-.jpg, (Public Domain) via Commons Wikimedia
2. “Melting point of the elements (K)” By Albris – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26z0aisqWVhYq6vsIygqaitoGJ%2FbrHLnqSepqSofA%3D%3D