Difference Between Gravimetric and Titrimetric Analysis
Table of Contents
The key difference between gravimetric and titrimetric analysis is that gravimetric analysis measures the quantity of an analyte using weight, whereas titrimetric analysis measures the quantity of an analyte using volume.
An analysis is a technique where we can measure the amount of an unknown compound with the use of a known amount of a known compound. We can take this amount as a volume or as a weight. If we measure volume, we call it a “volumetric analysis” or a “titrimetric analysis”. If we measure weight, we call it “gravimetric analysis”.
CONTENTS
1. Overview and Key Difference
2. What is Gravimetric Analysis
3. What is Titrimetric Analysis
4. Side by Side Comparison – Gravimetric vs Titrimetric Analysis in Tabular Form
5. Summary
What is Gravimetric Analysis?
Gravimetric analysis is a technique that comes under quantitative analysis where we can determine the weight of an unknown compound in a sample. In this method, the major step is the precipitation reactions, which leads to the separation of the desired compound from a given sample. A precipitation reaction can convert a dissolved compound into a precipitate which we can measure. If the sample is a mixture of several solids, we can first dissolve the sample in a suitable solvent and then we can add a suitable reagent that can precipitate the compound we need. We call it a precipitating agent. Eventually, we can separate the precipitate via filtration and measure its weight.

Figure 01: An Analytical Balance Used to Measure Minute Weights
Most importantly, the precipitating agent should precipitate only the required compound. In addition to this, the filtration should wash off all the other constituents other than the required compound. For the removal of unwanted constituents still present on the precipitate, we can wash the precipitate using water or any other solvent that does not dissolve the precipitate. Then we can dry the precipitate and weigh.
What is Titrimetric Analysis?
Titrimetric analysis is a type of quantitative analysis in which we can measure the amount of an unknown compound using its volume. In this method, we can use a titration for this determination, which leads to its name “titrimetric analysis”. Here, we use a second solution or reagent for the determination of the volume of the unknown compound present in a sample. Through the determination of the volume of the unknown, we can determine the concentration of that compound in the sample.

Figure 02: A Titration
We need several components for the experimental system when performing a titration. These include a burette, burette holder, a beaker or an Erlenmeyer flask and pipettes. Usually, we need to fill the reagent (having a known concentration) into the burette and take the sample (containing the unknown compound) into the beaker (a known volume). Furthermore, we can use indicators for the determination of the endpoint of the titration. It’s important to choose the correct indicator for a particular titration according to the pH range in which we perform the titration. For example, the indicator phenolphthalein works at the pH range of 8.3-10.0. The indicator gives a colour change at the endpoint. Ex: the colour of phenolphthalein at pH 8.3 is colourless, and at pH 10.0, it shows a pale pink colour.
What is the Difference Between Gravimetric and Titrimetric Analysis?
An analysis is a technique from which we can measure the amount of an unknown compound with the use of a known amount of a known compound. Gravimetric and titrimetric analysis are two such types of analysis processes. The key difference between gravimetric and titrimetric analysis is that gravimetric analysis measures the quantity of an analyte using weight, whereas titrimetric analysis measures the quantity of an analyte using volume.
The below infographic summarizes the differences between gravimetric and titrimetric analysis in tabular form.
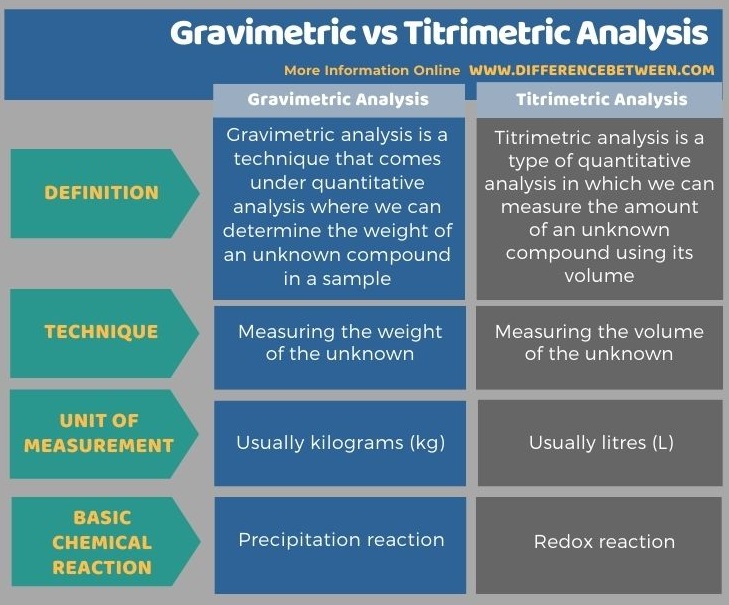
Summary – Gravimetric vs Titrimetric Analysis
An analysis is a technique from which we can measure the amount of an unknown compound with the use of a known amount of a known compound. Gravimetric and titrimetric analysis are two such types of analysis processes. The key difference between gravimetric and titrimetric analysis is that gravimetric analysis measures the quantity of an analyte using weight, whereas titrimetric analysis measures the quantity of an analyte using volume.
Reference:
1. “Titration.” LibreText 14 July 2020, Available here.
Image Courtesy:
1. “Analytical balance mettler ae-260” By US DEA – (Public Domain) via Commons Wikimedia
2. “School level titration demonstration” By UCL – Flickr (CC BY 2.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26z0ZqtoqWVqb%2Bqr4yapZ1lpJ7Bs7XMnquroZNirq%2Bty7Kqoqtf