Difference Between Fluorescence and Phosphorescence
Table of Contents
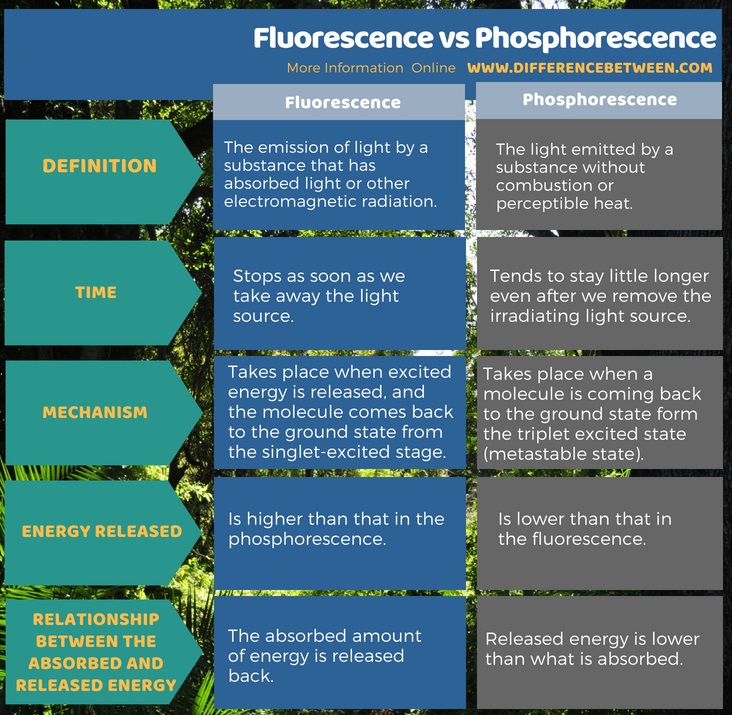
The key difference between fluorescence and phosphorescence is that Fluorescence stops as soon as we take away the light source whereas phosphorescence tends to stay little longer even after the irradiating light source is removed.
When a molecule or atom absorbs energy, it can undergo various changes. Fluorescence and phosphorescence are two such processes. In addition to the above key difference, there are some other differences between the two terms such as the energy released in the fluorescence process is higher than that in the phosphorescence.
CONTENTS
1. Overview and Key Difference
2. What is Fluorescence
3. What is Phosphorescence
4. Side by Side Comparison – Fluorescence vs Phosphorescence in Tabular Form
5. Summary
What is Fluorescence?
Electrons in an atom or a molecule can absorb the energy in the electromagnetic radiation and thereby excite to an upper energy state. This upper energy state is unstable; therefore, electron likes to come back to the ground state. When coming back, it emits the absorbed wavelength. In this relaxation process, they emit excess energy as photons. We call this relaxation process as fluorescence. Fluorescence takes place much more rapidly. Generally, it completes in about 10-5 seconds or less time from the time of excitation.
When gaseous atoms undergo fluorescence, atomic fluorescence takes place when exposed to radiation with a wavelength that exactly matches one of the absorption lines of the element. For example, gaseous sodium atoms absorb and excite by absorbing 589 nm radiations. Relaxation takes place after this by reemission of fluorescent radiation of the identical wavelength. Because of this, we can use fluorescence to identify different elements. When excitation and reemission wavelengths are the same, we call the resulting emission as resonance fluorescence.
Other Mechanisms
Other than fluorescence, there are other mechanisms by which an excited atom or molecule can give up its excess energy and relax to its ground state. Non-radiative relaxation and fluorescence emissions are two such important mechanisms. Because of many mechanisms, the lifetime of an excited state is brief. The relative number of molecules that fluoresce is small because this phenomenon requires structural features that slow the rate of the non-radiative relaxation and enhance the rate of fluorescence. In most molecules, these features are not there; therefore, they undergo non-radiative relaxation, and fluorescence does not occur. Molecular fluorescence bands are consisting of a large number of closely spaced lines; therefore, usually it is hard to resolve.
What is Phosphorescence?
When molecules absorb light and go to the excited state they have two options. They can either release energy and come back to the ground state immediately or undergo other non-radiative processes. If the excited molecule undergoes a non-radiative process, it emits some energy and come to a triplet state where the energy is somewhat lesser than the energy of the exited state, but it is higher than the ground state energy. Molecules can stay a bit longer in this less energy triplet state.

Figure 01: Phosphorescence
We call this state as the metastable state. Then metastable state (triplet state) can slowly decay by emitting photons, and come back to the ground state (singlet state). When this happens we call it phosphorescence.
What is the Difference Between Fluorescence and Phosphorescence?
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation while phosphorescence refers to the light emitted by a substance without combustion or perceptible heat. When we supply light to a sample of molecules, we immediately see the fluorescence. Fluorescence stops as soon as we take away the light source. But phosphorescence tends to stay little longer even after we remove the irradiating light source.
Summary – Fluorescence vs Phosphorescence
Both fluorescence and phosphorescence are chemical processes in which light absorption and emission occurs. The difference between fluorescence and phosphorescence is that Fluorescence stops as soon as we take away the light source whereas phosphorescence tends to stay little longer even after the irradiating light source is removed.
Reference:
1. “Fluorescence.” Wikipedia, Wikimedia Foundation, 3 June 2018. Available here
2. “Phosphorescence.” Wikipedia, Wikimedia Foundation, 28 May 2018. Available here
Image Courtesy:
1.’Phosphorescence’By Lưu Ly – Own work, (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26yy66mq52jmLKvr8RmmKecXavAbrzHqKqpoJ%2BnsrSvxKeanmc%3D