Difference Between Ferrous Sulfate and Iron Glycinate
Table of Contents
The key difference between ferrous sulfate and iron glycinate is that ferrous sulfate is less absorbed and more toxic than iron glycinate when used in medications.
Both ferrous sulfate and iron glycinate are useful as iron supplements. However, they have different properties, and their iron content is also different. Normally, we get iron from the food we eat. But, if the body doesn’t get enough iron, then we get iron deficiency anemia. Ferrous sulfate and iron glycinate are medications to treat this disease.
CONTENTS
1. Overview and Key Difference
2. What is Ferrous Sulfate
3. What is Iron Glycinate
4. Side by Side Comparison – Ferrous Sulfate vs Iron Glycinate in Tabular Form
5. Summary
What is Ferrous Sulfate?
Ferrous sulfate is a type of iron supplement that denotes a range of salts with the chemical formula FeSO4.xH2O. It is useful to prevent low levels of iron in the blood. Most commonly, it occurs in the heptahydrate form. Also, it has a blue-green appearance. Apart from its medicinal applications, it has industrial uses as well.
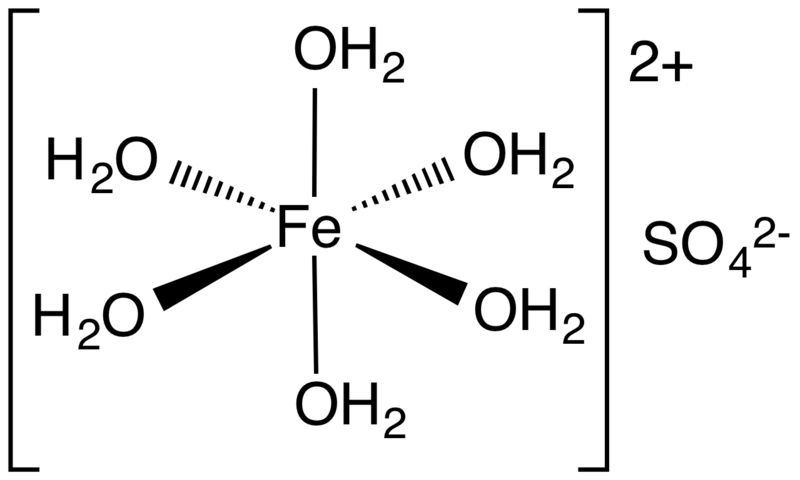
Figure 01: Structure of Ferrous Sulfate
When considering the production of this compound, it forms as a byproduct during the finishing of steel prior to plating or coating. Here, the steel sheet is passed through pickling baths of sulfuric where the ferrous sulfate formation occurs. Moreover, this compound can form in large amounts during the production of titanium dioxide from ilmenite using the sulfate process.
As a medication, physicians often recommend ferrous sulfate for iron deficiency though it is not the best option. It is less absorbed and also toxic. Furthermore, it can cause several side effects such as nausea, vomiting, stomach pains, constipation, etc.
What is Iron Glycinate?
Iron glycinate is a type of iron supplement that is better than other iron supplements. Our body absorbs this compound easily, and it is also comparatively less toxic. That means; it has fewer side effects and is more bioavailable. However, it causes a few gastrointestinal symptoms. The benefits of using iron glycinate include providing essential micronutrients required by all animals, high efficiency, good solubility and high absorption, easy handling, high bioavailability, etc.
What is the Difference Between Ferrous Sulfate and Iron Glycinate?
Ferrous sulfate is a type of iron supplement which denotes a range of salts with the chemical formula FeSO4.xH2O. Iron glycinate is a type of iron supplement which is better than other iron supplements. The key difference between ferrous sulfate and iron glycinate is that ferrous sulfate is less absorbed and more toxic than iron glycinate when used in medications.
Moreover, there is a difference between ferrous sulfate and iron glycinate in terms of their side effects. That is; ferrous sulfate has side effects such as nausea, vomiting, stomach pains, constipation, etc., while for iron glycinate, there are only a few side effects, including gastrointestinal symptoms. Hence, the efficiency of ferrous sulfate is lower than iron glycinate.
Below infographic summarizes the difference between ferrous sulfate and iron glycinate.
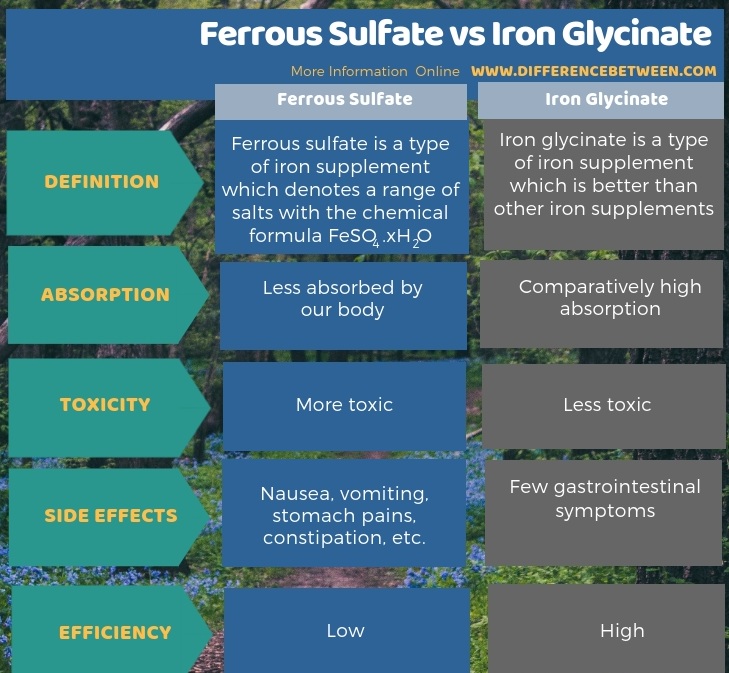
Summary – Ferrous Sulfate vs Iron Glycinate
The key difference between ferrous sulfate and iron glycinate is that ferrous sulfate is less absorbed and more toxic than iron glycinate when used in medications. Therefore, ferrous sulfate is less efficient than iron glycinate.
Reference:
1. “Ferrous Sulfate Oral : Uses, Side Effects, Interactions, Pictures, Warnings & Dosing.” WebMD, WebMD, Available here.
2. “Iron Deficiency Treatment for Chronic Fatigue Symptoms.” University Health News, 5 Mar. 2019, Available here.
Image Courtesy:
1. “Fe(H2O)6SO4” By Smokefoot – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26yxKupqK2jYsC2uMWaq55lkaOxbrXRqKVmn5yusKq6wK2caA%3D%3D