Difference Between Esterification and Neutralization
Table of Contents
The key difference between esterification and neutralization is that esterification produces an ester from an acid and an alcohol, whereas neutralization produces a salt from an acid and a base.
Esterification and neutralization are two important reactions of chemistry. Esterification, as its name implies, is a chemical reaction which produces an ester at the end of the reaction. Neutralization refers to the balancing of acidity from alkalinity.
CONTENTS
1. Overview and Key Difference
2. What is Esterification
3. What is Neutralization
4. Side by Side Comparison – Esterification vs Neutralization in Tabular Form
5. Summary
What is Esterification?
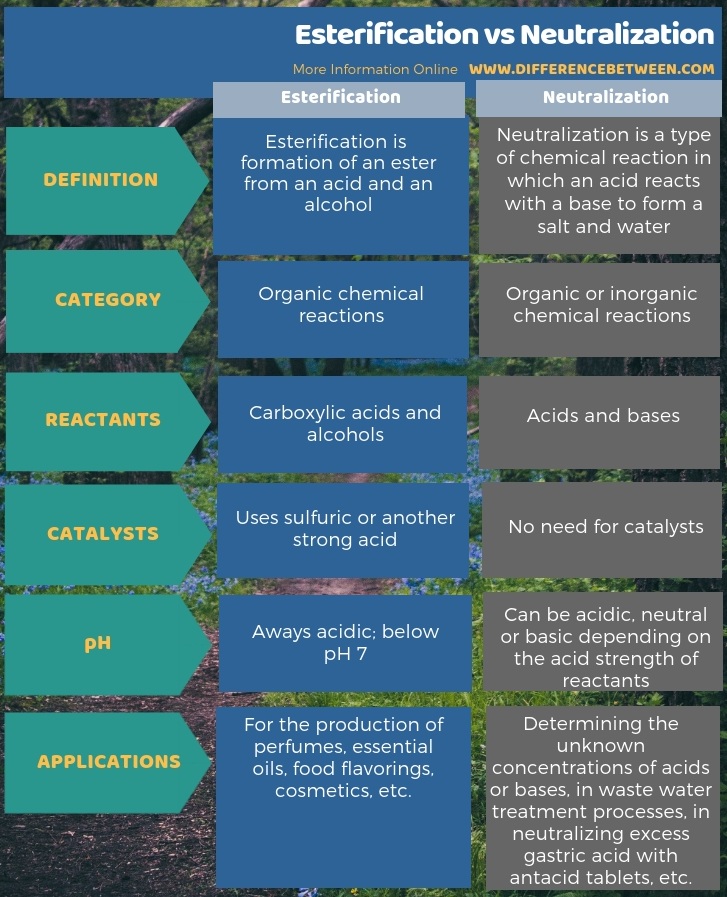
Esterification is the process of forming an ester from an acid and an alcohol. The acid is usually a carboxylic acid, and the alcohol has to be a primary or secondary alcohol. And, the reaction takes place in acidic environments. Thus, we use sulfuric acid as a strong acid for the reaction. It acts as a catalyst for the reaction because a mixture of carboxylic acid and alcohol gives nothing if the medium is not acidic. As a byproduct, water molecules are formed. Therefore, this is a condensation reaction.
The pi bond in the carbonyl group of the carboxylic acid can act as a base because of the distortion of the electrons due to the difference in electronegativity between oxygen and carbon atoms. The electrons in the pi bond are given to one hydrogen atom in the sulfuric acid molecule. Thus, this converts the –C=O bond into –C-OH.
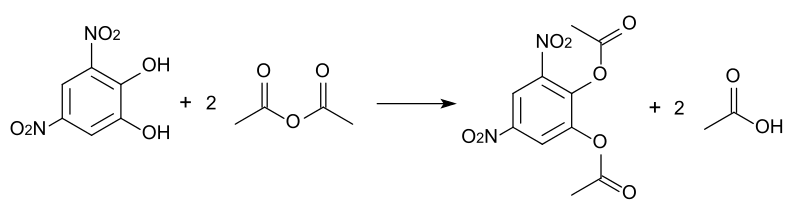
Figure 01: An Example of an Esterification Reaction
Here, the carbon atom has a positive charge because it has only three chemical bonds around it. We call this a carbocation. In the presence of an alcohol, the lone electron pairs in the oxygen atom of the alcohol can give electrons to the carbon atom of the carbocation. Therefore, the alcohol acts as a nucleophile. Afterwards, rearrangements occur and form an ester and a water molecule.
What is Neutralization?
Neutralization is a type of chemical reaction in which an acid reacts with a base to form a salt and water. Therefore, this reaction involves the combination of H+ ions and OH– ions, and it generates water. Hence, there is no hydrogen ions or hydroxide ions in excess in the reaction mixture after the completion of the reaction.

If a strong acid reacts with a strong base, then the pH of the final reaction mixture is 7. Other than that, the pH of the reaction mixture depends on the acid strength of the reactants. When considering the applications of neutralization, it is important in determining the unknown concentrations of acids or bases, in wastewater treatment processes, in neutralizing excess gastric acid with antacid tablets, etc.
What are the Similarities Between Esterification and Neutralization?
- Both reactions produce water as a byproduct
- Both reactions involve the combination of H+ ions and OH–
What is the Difference Between Esterification and Neutralization?
Esterification and neutralization are important reactions in chemistry. The key difference between esterification and neutralization is that esterification produces an ester from an acid and an alcohol, whereas neutralization produces a salt from an acid and a base. Furthermore, the reactants for esterification are carboxylic acid and alcohols while for neutralization, the reactants are acids and bases.
Moreover, another difference between esterification and neutralization is that esterification requires a catalyst such as sulfuric acid while neutralization does not require any catalyst.
Summary – Esterification vs Neutralization
Esterification and neutralization are important reactions in chemistry. In summary, the key difference between esterification and neutralization is that esterification produces an ester from an acid and an alcohol, whereas neutralization produces a salt from an acid and a base.
Reference:
1. “Mechanism of Esterification (Organic Chemistry) – ChemistryScore.” Learn Chemistry Here, 19 Sept. 2018, Available here.
2. Admin. “Ester – Process of Esterification with Structure, Properties & Uses.” BYJUS, Byju’s, 25 July 2018, Available here.
Image Courtesy:
1. “Esterification 3,5-Dinitrocatechol” By MegaByte07 – Own work (CC BY-SA 3.0) via Commons Wikimedia
2. “Titolazione” By Luigi Chiesa – Draw by Luigi Chiesa (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26x0q2cq6GWnrCiwMiopWaZnpl6r7HUramapJmvrrW1zqdm