Difference Between Differential Rate Law and Integrated Rate Law
Table of Contents
Key Difference – Differential Rate Law vs Integrated Rate Law
Differential rate law and integrated rate law are two forms of rate laws. The key difference between differential rate law and integrated rate law is that differential rate law gives the rate of a chemical reaction as a function of the change in concentration of one or more reactants during a particular time period whereas integrated rate law gives the rate of a chemical reaction as a function of the initial concentration of one or more reactants after a specific period of time.
The reaction rate is the measure of the change of concentration of reactants or products during the progression of a chemical reaction. Different rate laws are used to explain the reaction progress. These rate laws are expressed as mathematical relationships between different parameters.
CONTENTS
1. Overview and Key Difference
2. What is Differential Rate Law
3. What is Integrated Rate Law
4. Relationship Between Differential Rate Law and Integrated Rate Law
5. Side by Side Comparison – Differential Rate Law vs Integrated Rate Law in Tabular Form
6. Summary
What is Differential Rate Law?
The differential rate law is used to determine the rate of a chemical reaction as a function of the change in concentration of one or more reactants during a particular time period. The differential rate law indicates what is happening at the molecular level of a chemical reaction. The overall mechanism of a chemical reaction can be determined using differential rate laws (conversion of reactants into products).
Differential Rate Law Equation
The differential rate law for the below chemical reaction can be given as a mathematical expression.
A → B + C
Rate = – {d[A] / dt} = k[A]n
Here, [A] is the concentration of reactant “A” and “k” is the rate constant. “n” gives the order of reaction. The differential rate law equation can be integrated to obtain a clear relationship between [A] and time “t”. This integration gives the integrated rate law.
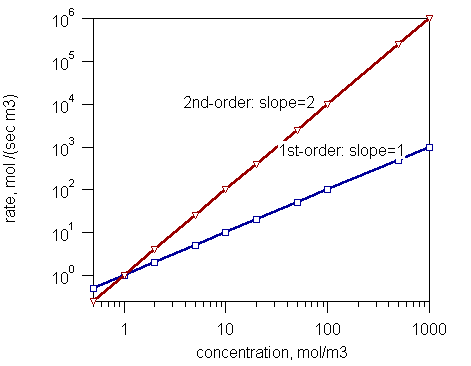
Figure 1: A Graph showing Order of Reaction
What is Integrated Rate Law?
The integrated rate law gives the rate of a chemical reaction as a function of the initial concentration of one or more reactants after a specific period of time. The integrated rate law can be used to determine the rate constant of a particular chemical reaction, and the reaction order can be obtained via experimental data.
Integrated Rate Law Equation
For the chemical reaction A → B + C, integrated rate law can be expressed as a mathematical expression as given below.
ln[A] = -kt + ln[A]0
Here, [A]0 is the initial concentration of the reactant A and [A] is the concentration of reactant “A” after “t” time has passed. However, integrated rate laws are different from each other based on the order of the reaction “n”. The above equation is given for zero order chemical reactions.
For first order reactions, the rate law equation is,
[A] = [A]e-kt
For second order reactions, the rate law equation is,
1/[A] = 1/[A]0 + kt
In order to determine the rate constant of a reaction, above equations can be used as follows.
For first order reactions,
k = {ln[A] – ln[A]0} / t
For second order reactions,
k = {1/[A] – 1/[A]0} / t
What is the Relationship Between Differential Rate Law and Integrated Rate Law?
- The differential rate law of a chemical reaction can be integrated to obtain the integrated rate law of the same chemical reaction.
What is the Difference Between Differential Rate Law and Integrated Rate Law?
Differential Rate Law vs Integrated Rate Law | |
| Differential rate law is used to determine the rate of a chemical reaction as a function of the change in concentration of one or more reactants during a particular time period. | Integrated rate law gives the rate of a chemical reaction as a function of the initial concentration (or the concentration at a particular moment) of one or more reactants after a specific period of time. |
| Application | |
| Differential rate law can be used to indicate what is happening at the molecular level of a chemical reaction and, the overall mechanism of a chemical reaction can be determined using this rate law. | Integrated rate law can be used to determine the rate constant of a particular chemical reaction. |
| Usage | |
| Differential rate law is difficult to use when compared with integrated rate law. | Integrated law makes it easy to determine the clear relationship between the concentration of reactants and the time elapsed. |
Summary – Differential Rate Law vs Integrated Rate Law
Rate law of a chemical reaction gives the relationship between reaction rate and the concentrations of reactants. The key difference between differential rate law and integrated rate law is that the differential rate law gives the rate of a chemical reaction as a function of the change in concentration of one or more reactants during a particular time period whereas integrated rate law gives the rate of a chemical reaction as a function of the initial concentration of one or more reactants after a specific period of time.
Reference:
1. “Rate Laws – Differential.” Chemistry LibreTexts, Libretexts, 17 July 2015, Available here.
2. Libretexts. “12.4: Integrated Rate Laws.” Chemistry LibreTexts, Libretexts, 11 Sept. 2017, Available here.]
Image Courtesy:
1. “Rateloglogplot” By Fabiuccio~enwikibooks at English Wikibooks – Transferred from en.wikibooks to Commons.(Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26wyJ%2BdnqqVo8GqrctmqZqslWK5osOMmqWdZaaoeqq6056eq5mkmrFuvsCtnGakkax8