Difference Between DCC and EDC
Table of Contents
The key difference between DCC and EDC is that DCC is a cyclic compound, whereas EDC is an aliphatic compound.
DCC and EDC are organic compounds. The term DCC stands for N,N′-Dicyclohexylcarbodiimide while the term EDC stands for 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide. Both these are imides, meaning these compounds have a –N=C=N- bond, which represents the functional properties of imides.
CONTENTS
1. Overview and Key Difference
2. What is DCC
3. What is EDC
4. Side by Side Comparison – DCC vs EDC in Tabular Form
5. Summary
What is DCC?
The term DCC stands for N,N′-Dicyclohexylcarbodiimide. It is also abbreviated as DCCD. The chemical formula of this compound is (C6H11N)2C. Furthermore, it is an organic compound having the general structure of an imide. It can be produced as a waxy white solid that has a sweet odour. Concerning the properties, this solid substance has a very low melting point; thus, it melts easily, making itself easy to handle. Also, this substance is highly soluble in solvents such as dichloromethane, tetrahydrofuran, acetonitrile, and dimethylformamide. However, it is insoluble in water.
When considering the chemical structure of this compound, it has a linear C-N=C=N-C structure at the centre of the molecule. Therefore, this structure is related to the chemical structure of allene. There are several methods of producing DCC. One method includes the use of palladium acetate, iodine, and oxygen for the coupling of cyclohexyl amine and cyclohexyl isocyanide. This reaction gives about 67% DCC. Another method includes the use of dicyclohexylurea in the presence of a phase transfer catalyst. However, this second method gives only 50% yield of DCC.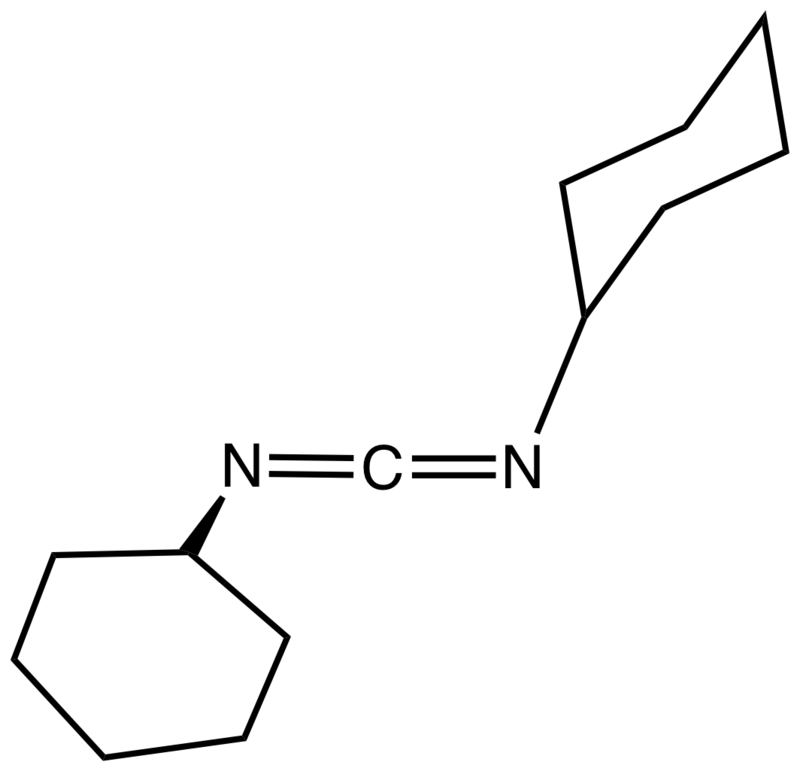
DCC is an important dehydrating agent for the production of amides, ketones, and nitriles. Here, the DCC molecule becomes hydrated into dicyclohexylurea or DCU. The resultant compound is insoluble in many organic solvents and water, so we can readily remove it via filtration. Moreover, DCC is important in converting secondary alcohols.
Besides, the DCC is known as an inhibitor for ATP synthase. However, the primary use of DCC is the coupling of amino acids during the process of artificial protein synthesis. Despite all the important applications of DCC, it should be handled carefully because it is a potent allergen and a sensitizer. It often causes skin rashes.
What is EDC?
The term EDC stands for 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide. The chemical formula of EDC is C8H17N3. It is also abbreviated as EDAC or EDCI. It is a water-soluble carbodiimide. Typically, a solution of EDC has a pH range of 4.0 to 6.0. Generally, we can use this compound as a carboxyl activating agent for the coupling of primary amines to yield amide bonds.

EDC is a commercially available organic compound that can be produced via coupling ethyl isocyanate with N,N-dimethylpropane-1,3-diamine. This reaction produces urea which then can be converted into EDC via dehydration. When considering the applications of EDC, most common uses for this carbodiimide include peptide synthesis, protein crosslinking to nucleic acids, and preparation of immunoconjugates.
What is the Difference Between DCC and EDC?
DCC stands for N,N′-Dicyclohexylcarbodiimide while EDC stands for 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide. The key difference between DCC and EDC is that DCC is a cyclic compound, whereas EDC is an aliphatic compound. Another major difference between DCC and EDC is that DCC is insoluble in water while EDC is water-soluble.
Moreover, when considering the uses of these two compounds, DCC is useful as the coupling of amino acids during the process of artificial protein synthesis while EDC is useful for peptide synthesis, protein crosslinking to nucleic acids, and in the preparation of immunoconjugates.
Below infographic summarizes the difference between DCC and EDC.

Summary – DCC vs EDC
DCC and EDC are organic compounds, and DCC stands for N,N′-Dicyclohexylcarbodiimide while EDC stands for 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide. The key difference between DCC and EDC is that DCC is a cyclic compound, whereas EDC is an aliphatic compound.
Reference:
1. 1-Ethyl-3-(3-Dimethylaminopropyl)Carbodiimide. 23 June 2020, Available here.
Image Courtesy:
1. “Dicyclohexylurea, 3-d” By Smokefoot – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “EDC Structure” By ~K – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26wwpxkmqaUYrKlr44%3D