Difference Between Critical Point and Triple Point
Table of Contents
Key Difference – Critical Point vs Triple Point
Critical point and triple point are terms used to explain temperatures and pressures at which two or more phases of substances can coexist with each other. The critical point is the condition at which the liquid and vapour phase of the same substance coexist. The triple point is the condition at which all three phases of matter can coexist with each other. The key difference between a critical point and the triple point is that critical point describes the coexistence of two phases of same substance whereas triple point describes the coexistence of three phases of the same substance.
CONTENTS
1. Overview and Key Difference
2. What is Critical Point
3. What is Triple Point
4. Similarities Between Critical Point and Triple Point
5. Side by Side Comparison – Critical Point vs Triple Point in Tabular Form
6. Summary
What is Critical Point?
The critical point of a substance is the end point of the phase equilibrium curve of that substance. A phase equilibrium curve or a phase diagram is the graph of pressure versus temperature in which the phase changes of the substance is shown. This shows the temperatures and pressure at which the substance exists as a solid, liquid or gas. The critical point is the temperature and pressure at which the liquid and vapour phase coexist.
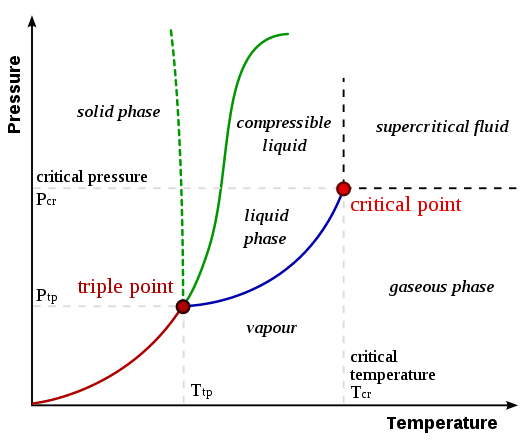
Figure 01: A Phase Diagram showing Both Critical Point and Triple Point
The temperature and pressure at critical point are named as critical temperature (Tc) and critical pressure (Pc). As shown in the above image, the lines between two phases are known as boundaries. A critical point indicates the point at which line boundaries vanish.
Knowing the critical point of a substance is sometimes very important. For example, a gas can never be condensed at temperatures and pressures above its critical point. This is because the intermolecular forces between gas molecule are weakened at very high temperatures since the kinetic energy of those molecules is increased.
There are two types of the critical point;
Liquid-Vapour Critical Point
This is a typical critical point at which the vapour of a substance is coexisting with its liquid form. The critical point of water is at 647 K and 22.064 MPa.
Liquid-Liquid Critical Point
This type of critical points is defined for solutions. It is the temperature and pressure at which a solution mixture is separated into two distinct liquid phases.
What is Triple Point?
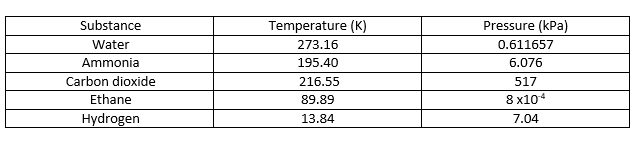
The triple point is the temperature and pressure at which solid, liquid, and vapour phases of a particular substance coexist in equilibrium. It describes a specific thermodynamic state of matte. Sometimes, the triple point may involve more than one solid phase when there are polymorphs of the substance exist. In a phase diagram, the triple point is the point at which all three boundary lines meet with each other. Some examples of triple points are given below.
What are the Similarities Between Critical Point and Triple Point?
- Both Critical Point and Triple Point describe certain specific temperatures and pressures.
- Both Critical Point and Triple Point describe equilibrium states where two or more physical states of a substance coexist.
What is the Difference Between Critical Point and Triple Point?
Critical Point vs Triple Point | |
| The critical point of a substance is the end point of the phase equilibrium curve of that substance. | The triple point is the temperature and pressure at which solid, liquid, and vapour phases of a particular substance coexist in equilibrium. |
| Phases | |
| Critical point describes the coexistence of two phases of the same substance. | Triple point describes the coexistence of three phases of the same substance. |
| Water as an Example | |
| The critical point of water is at 647 K and 22.064 MPa. | The triple point of water is at 273.16 K and 0.611657 MPa. |
| Phase Diagram | |
| The critical point is the end point of a phase diagram curve. | Triple point is the point at which all the boundary line meet with each other. |
Summary – Critical Point vs Triple Point
Critical point of a substance is the end point of the phase equilibrium curve of that substance which gives the temperature and pressure at which liquid and vapour phase of a substance can coexist with each other. The triple point gives the temperature and pressure at which all three phases of matter can coexist with each other. The difference between a critical point and the triple point is that critical point describes the coexistence of two phases of same substance whereas triple point describes the coexistence of three phases of the same substance.
Reference:
1.Helmenstine, Anne Marie, D. “Triple Point Definition and Example (Chemistry).” ThoughtCo, Nov. 10, 2017. Available here
2.“Triple point.” Wikipedia, Wikimedia Foundation, 6 Mar. 2018. Available here
3.“Critical Point.” Chemistry LibreTexts, Libretexts, 21 July 2016. Available here
Image Courtesy:
1.’Phase-diag2′ By Matthieumarechal, (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26v0aKropuRoXqxu8inq2aZnpl6t7%2BMramiqJyaerG7yKeraA%3D%3D