Difference Between Covalent and Noncovalent Bonds
Table of Contents
The key difference between covalent and noncovalent bonds is that covalent bonds form when two atoms share their electrons with each other whereas noncovalent bonds form either by completely exchanging electrons between two atoms or by not exchanging any electron.
There are four major types of chemical bonds: covalent bonds, ionic bonds, hydrogen bonds, and Van der Waals interactions. When we categorize chemical bonds as covalent and noncovalent bonds, ionic, hydrogen bonds, and Van der Waals interactions fall under the category of noncovalent bonds.
CONTENTS
1. Overview and Key Difference
2. What are Covalent Bonds
3. What are Noncovalent Bonds
4. Side by Side Comparison – Covalent vs Noncovalent Bonds in Tabular Form
5. Summary
What are Covalent Bonds?
A covalent bond is a type of chemical bond that forms when two atoms share an electron pair between them. It is named as a “molecular bond”. These bonds form when “shared pairs” or “bonding pairs” exist between atoms. A covalent bond forms due to the stable balance of attractive and repulsive forces between atoms when they share electrons. Sharing electrons between atoms allows each atom to have the equivalent of a full outer shell. Usually, this type of bond forms between two nonmetal atoms having nearly similar electronegativity values or between an electron and a positively charged metal ion.
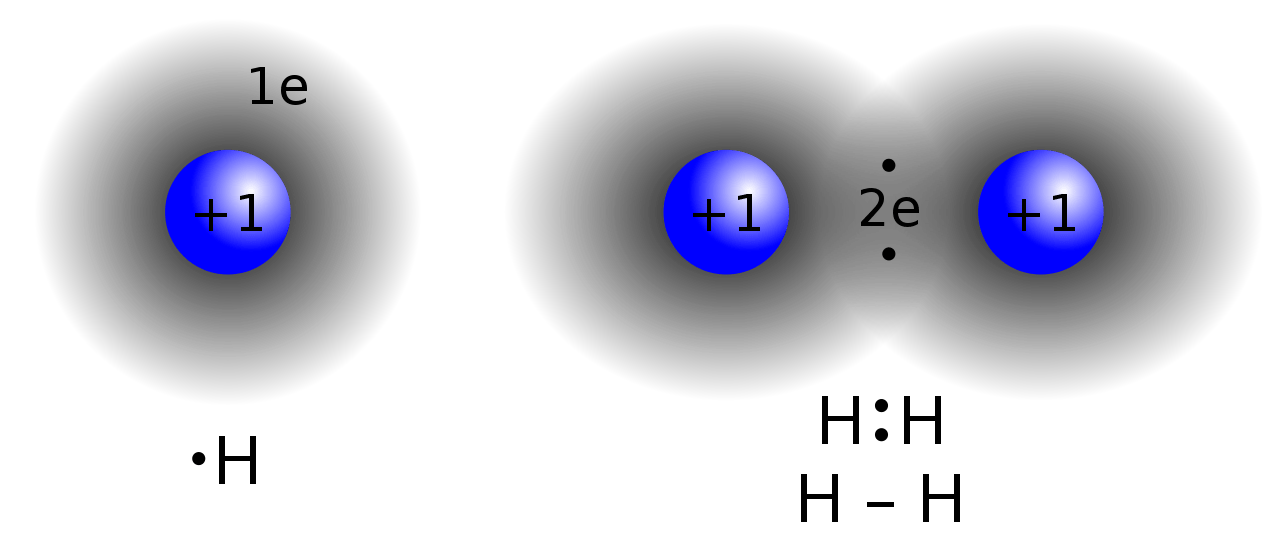
There are two major types of covalent bonds: polar covalent bonds and nonpolar covalent bonds. Polar covalent bonds exist between two atoms with a difference between their electronegativity values in the range of 0.4 to 1.7. Nonpolar covalent bonds form if this difference is lower than 0.4. Here, a high difference between electronegativity values means, one atom (having the higher electronegativity value) attracts the electrons more than the other atom, making the bond polar.
According to the number of electron pairs that are being shared between two atoms, we can identify three major types of covalent bonds as single bonds, which involve one electron pair, double bonds, which involve two electron pairs, and a triple bond, which involves three electron pairs.
What are Noncovalent Bonds?
Noncovalent bonds are chemical bonds that form either by completely exchanging electrons between atoms or by not exchanging electrons at all. There are three types of noncovalent bonds as ionic bonds, hydrogen bonds, and Van der Waals interactions.
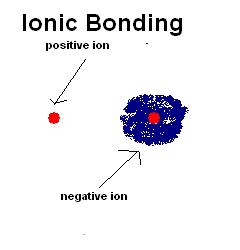
An atom can gain or lose electrons and form negative or positive charged particles in order to get a stable electron configuration. We call these particles “ions”. They have electrostatic interactions between them. An ionic bond can be described as the attraction force between these oppositely charged ions. The electrostatic interaction between ions is influenced by the electronegativity of the atoms in the ionic bond. Therefore, electronegativity gives a measurement of the atoms’ affinity for electrons. An atom with high electronegativity can attract electrons from an atom with low electronegativity to form an ionic bond.
Hydrogen bonds are another noncovalent bond. It is a type of attraction force between two atoms of two different molecules which is a weak attraction force. However, when comparing with other types of intramolecular forces such as polar-polar interactions, nonpolar-nonpolar interactions like Vander Waal forces, hydrogen bonds are stronger. Usually, hydrogen bonds form between polar covalent molecules. These molecules contain polar covalent bonds, which form as a result of the difference in the electronegativity values of the atoms that are in the covalent bond.
Van der Waals interactions are another type of noncovalent bond. They are weak attraction forces between two atoms in two nonpolar molecules. A Van der Waals interaction is either an induced attraction or repulsion which is caused by correlations in the fluctuating polarizations of nearby particles.
What is the Difference Between Covalent and Noncovalent Bonds?
Covalent and noncovalent bonds are the two broad classes of chemical bonds in chemistry. Covalent bonds can be found in three more subgroups as ionic bonds, hydrogen bonds, and Van der Waals interactions. The key difference between covalent and noncovalent bonds is that covalent bonds form when two atoms share their electrons with each other whereas noncovalent bonds form either by completely exchanging electrons between two atoms or by not exchanging any electron.
Below infographic lists out the differences between covalent and noncovalent bonds in more detail.

Summary – Covalent vs Noncovalent Bonds
Covalent and noncovalent bonds are the two broad classes of chemical bonds in chemistry. covalent bonds can be found in three more subgroups as ionic bonds, hydrogen bonds, and Van der Waals interactions. The key difference between covalent and noncovalent bonds is that covalent bonds form when two atoms share their electrons with each other whereas noncovalent bonds form either by completely exchanging electrons between two atoms or by not exchanging any electron.
Reference:
1. “Van Der Waals Forces.” Chemistry LibreTexts, Libretexts, 15 Aug. 2020, Available here.
Image Courtesy:
1. “Covalent bond hydrogen” By Jacek FH – Own work (CC BY-SA 3.0) via Commons Wikimedia
2. “Ionic bonding” By Jayron32.talk.contribs – I created this work entirely by myself. (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vzq%2BYpZ2eqXqiusNmpaimk6TDorjEp6tmmp%2BjsbR7