Difference Between Cobalt and Titanium
Table of Contents
The key difference between cobalt and titanium is in their appearance; cobalt is a hard, lustrous bluish-gray metal whereas the titanium is a silvery gray-white metal.
Both cobalt and titanium are metals in the d-block. They mainly differ from each other according to their appearance. However, there are many more differences between cobalt and titanium. For example, when considering the magnetic properties of these metals, cobalt is ferromagnetic whereas titanium is paramagnetic. Let us discuss more differences between them.
CONTENTS
1. Overview and Key Difference
2. What is Cobalt
3. What is Titanium
4. Side by Side Comparison – Cobalt vs Titanium in Tabular Form
5. Summary
What is Cobalt?
Cobalt is a chemical element having the symbol Co and atomic number 27. It is a d-block element in the periodic table and is a metal. Therefore, it does not occur as the individual metal on earth’s crust, instead, occurs in combination with other elements. However, we can produce the free element via smelting process; It is a hard, lustrous bluish-gray metal.
The atomic mass of this element is 58.93 amu. It locates in the periodic table in the group 9 and period 4. Moreover, we can classify it as a transition metal. The electron configuration of this metal is [Ar] 3d7 4s2. At standard pressure and temperature, it is in the solid state. The melting point and boiling points are 1495 °C and 2927 °C respectively. The most common oxidation states of this metal are +2, +3 and +4. Its crystal structure is hexagonal close-packed structure.

Figure 01: Appearance of Cobalt
Moreover, cobalt is a ferromagnetic material. It means it is highly attracted to the magnets. The specific gravity of this metal is 8.9, which is a very high value. Halogens and sulfur can attack this metal. However, it is a weakly reducing metal. We can protect it via oxidation by a passivating oxide film.
When considering the production of cobalt, we can use ores of cobalt such as cobaltite, erythrite, glaucodot, and skutterudite. But, most of the times, manufacturers obtain this metal via reducing the cobalt byproducts of nickel and copper mining.
What is Titanium?
Titanium is a chemical element having the symbol Ti and atomic number 22. It is a metal in the d block. It has a silvery grey-white metallic appearance. Moreover, it is a transition metal. It has a high strength compared to its low density. More importantly, it is corrosion resistant upon sea water, aqua regia and chlorine.
The standard atomic weight is 47.86 amu. It locates in the group 4 and period 4 of the periodic table. The electron configuration is [Ar] 3d2 4s2. It exists in the solid state at standard temperature and pressure. The melting point and boiling points of this metal are 1668 °C and 3287 °C respectively. The most common and stable oxidation state of this metal is +4.

Figure 02: Appearance of Titanium
In addition to the fact that it has a high strength to weight ratio, the metal is quite ductile and lustrous. Due to its high melting point, titanium is important as a refractory material. Moreover, it is paramagnetic and has a low electrical and thermal conductivity. We can find this metal commonly as in oxide form in most igneous rocks and in the sediments derived from these rocks. It is the ninth most abundant element on earth’s crust. The common minerals which bear titanium metal include anatase, brookite, ilmenite, perovskite, rutile, and titanite.
What is the Difference Between Cobalt and Titanium?
Cobalt is a chemical element having the symbol Co and atomic number 27. On the other hand, titanium is a chemical element having the symbol Ti and atomic number 22. The atomic mass of this element is 58.93 amu while the atomic mass of titanium is 47.86 amu. Moreover, cobalt is a hard lustrous bluish-gray metal but, on the contrary, the titanium has a silvery grey-white metallic appearance. The infographic below presents more information on the difference between cobalt and titanium.
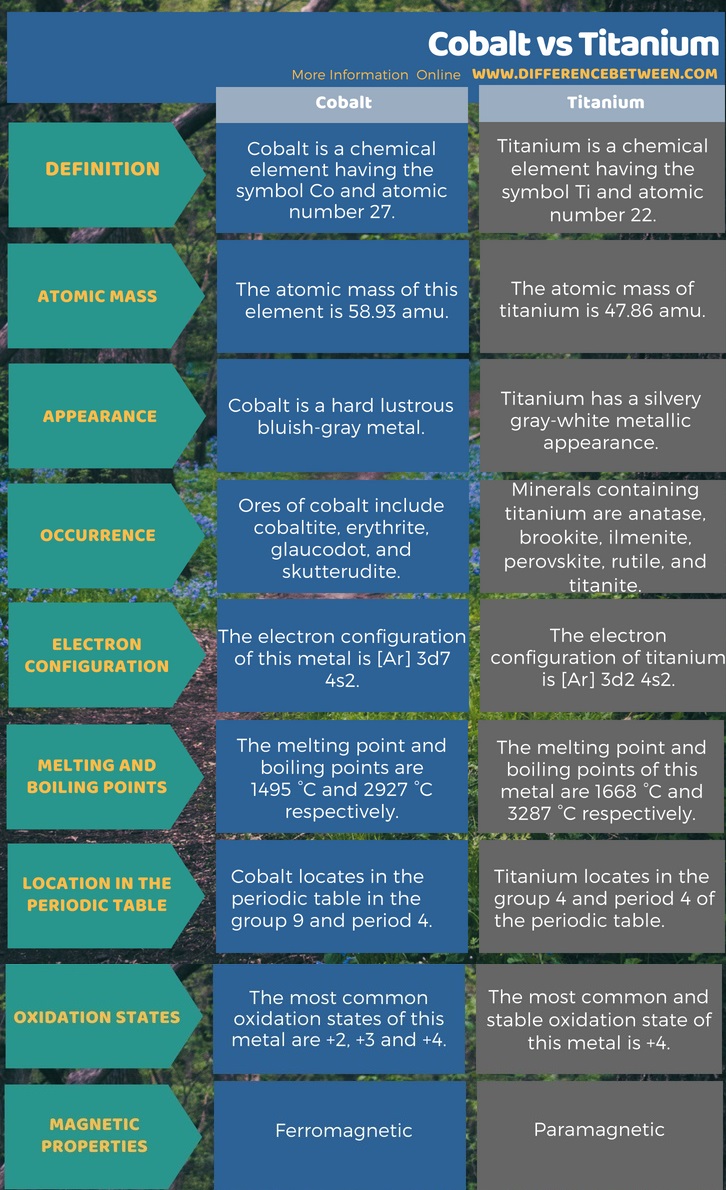
Summary – Cobalt vs Titanium
Cobalt and titanium are transition metals in the d block of the periodic table. The key difference between cobalt and titanium is in their appearance; cobalt is a hard lustrous bluish-gray metal whereas titanium is silvery grey-white metal.
Reference:
1. “Cobalt.” Wikipedia, Wikimedia Foundation, 25 July 2018. Available here
2. “Titanium.” Wikipedia, Wikimedia Foundation, 25 July 2018. Available here
Image Courtesy:
1.’Kobalt electrolytic and 1cm3 cube’By Alchemist-hp – Own work, (FAL) via Commons Wikimedia
2.’Titan-crystal bar’By Alchemist-hp (pse-mendelejew.de) – Own work, (CC BY-SA 3.0) via Common Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vzpuYpaxdlruledOiq5qmmaq6cA%3D%3D