Difference Between Chromate and Dichromate
Table of Contents
The key difference between chromate and dichromate is that chromate appears in a bright yellow colour, whereas dichromate appears in a bright orange colour.
Chromate and dichromate are anions containing chromium and oxygen atoms. Therefore, they are oxyanions of chromium. We often use these terms as general terms to name the compounds containing these anions. These two anions have closely similar chemical structures; chromate has one chromate anion whereas dichromate has two chromate anions in combination with each other. But they have different appearances.
CONTENTS
1. Overview and Key Difference
2. What is Chromate
3. What is Dichromate
4. Side by Side Comparison – Chromate vs Dichromate in Tabular Form
5. Summary
What is Chromate?
Chromate is an oxyanion of chromium having the chemical formula CrO42-. Generally, we use this term to name the compounds containing this anion collectively as one group, i.e. compounds containing the chromate anion are named as chromates. Usually, the chromates have a bright yellow colour. The chromium atom in this anion is in +6 oxidation state. It is a moderately strong oxidizing agent. The molar mass of this anion is 115.99 g/mol.
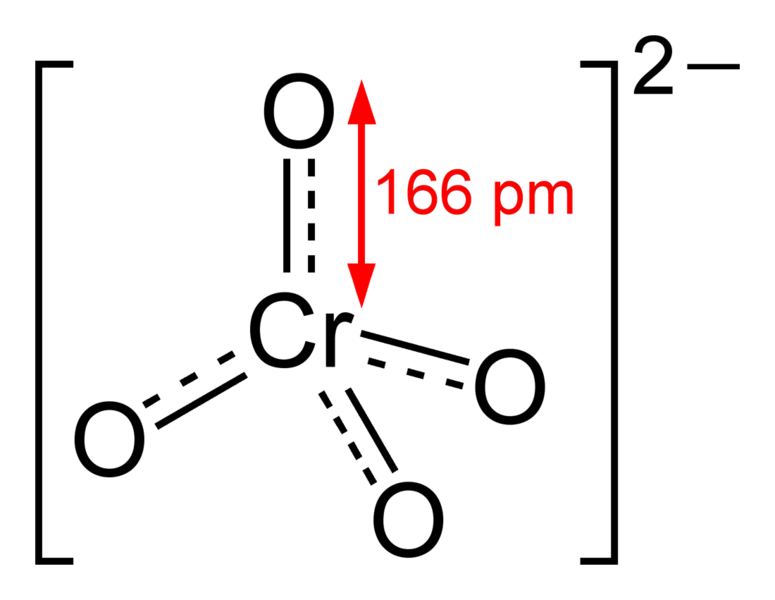
Figure 01: Chemical Structure of Chromate Ion
When considering the properties and reactions of chromates, they can react with hydrogen peroxide since the peroxide anion replaces one or more oxygen atoms. In an aqueous solution, there is usually an equilibrium between chromate and dichromate. However, we can find a high amount of chromate at high pH values (higher than 6.5 pH) where dichromate amount is very small. This means, in alkaline solutions, the predominant species is chromate.
What is Dichromate?
Dichromate is an oxyanion of chromium having the chemical formula Cr2O72-. Usually, we use this term to name the compounds containing this anion collectively as one group. For e.g., potassium dichromate, sodium dichromate are dichromates. Furthermore, the compounds containing dichromate as the anion show a bright orange colour. The molar mass of this anion is 215.99 g/mol. When considering the geometry of dichromate, it has tetrahedral geometry around the chromium atom.

Figure 02: Appearance of Dichromate Compounds
In an aqueous solution, normally there is an equilibrium between chromate and dichromate. However, we can find a high amount of dichromate and very small amount of chromate at low pH values (lower than 6.5 pH).
What is the Difference Between Chromate and Dichromate?
Chromate and dichromate are anions containing chromium and oxygen atoms. Therefore, they are oxyanions of chromium. The key difference between chromate and dichromate is that chromate appears in a bright yellow colour, whereas dichromate appears in a bright orange colour. Moreover, chromate ion has one chromium atom per anion while dichromate ion has two chromium atoms per anion.
Besides, a further difference between chromate and dichromate is in their molar mass. The molar mass of dichromate anion is 215.99 g/mol while the molar mass of chromate anion is 115.99 g/mol. In an aqueous solution, there is normally an equilibrium between chromate and dichromate. However, we can find a high amount of chromate at high pH values (higher than 6.5 pH) where dichromate amount is very small. But at low pH values (lower than 6.5 pH), there are more dichromate ions.

Summary – Chromate vs Dichromate
Chromate and dichromate are anions containing chromium and oxygen atoms. Therefore, they are oxyanions of chromium. The key difference between chromate and dichromate is that chromate appears in a bright yellow colour whereas dichromate appears in a bright orange colour. In an aqueous solution, normally there is an equilibrium between chromate and dichromate. However, we can find a high amount of chromate at high pH values (higher than 6.5 pH), while in low pH values (lower than 6.5 pH), there are more dichromate ions.
Reference:
1.“Dichromate.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, Available here.
2.“Potassium Dichromate.” Wikipedia, Wikimedia Foundation, 9 Nov. 2019, Available here.
3. Cheremisinoff, Paul N. “Treatment of Effluent Fertilizer Industry Example.” Waste Minimization and Cost Reduction for the Process Industries, 1995, pp. 285–324., doi:10.1016/b978-081551388-9.50010-5.
Image Courtesy:
1. “Chromate-2D-dimensions” By Ben Mills – Own work (Public Domain) via Commons Wikimedia
2. “Potassium-dichromate-sample” By Benjah-bmm27 – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vx6umppmkmnqiusNmm6KbmKe8rq3TnmY%3D