Difference Between Chloroform and Carbon Tetrachloride
Table of Contents
The key difference between chloroform and carbon tetrachloride is that the chloroform (CHCl3) is a powerful anaesthetic, but carbon tetrachloride (CCl4) is not an anaesthetic.
Furthermore, both chloroform and carbon tetrachloride has the same chemical geometry; tetrahedral geometry. Since the chemical structure and composition of carbon tetrachloride resembles chloroform, most people misunderstand thinking that both are the same. However, carbon tetrachloride has only carbon and chlorine atoms whereas chloroform has carbon, chlorine and hydrogen atoms.
CONTENTS
1. Overview and Key Difference
2. What is Chloroform
3. What is Carbon Tetrachloride
4. Side by Side Comparison – Chloroform vs Carbon Tetrachloride in Tabular Form
5. Summary
What is Chloroform?
Chloroform is CHCl3 , which is used as a powerful anaesthetic. The IUPAC name of this compound is trichloromethane. It is a colourless and dense liquid which has a sweet smell. The purpose of the large-scale production of this compound is as a precursor to produce PTFE. Most of the chloroform in the environment (about 90%) is due to emissions of natural origin. Ex: many types of seaweed and fungi produce this compound.
The molar mass of the compound is 119.37 g/mol, and it appears as a colourless liquid at room temperature. It has a heavy ethereal odour. The melting point is −63.5 °C, and the boiling point is 61.15 °C. Moreover, it decomposes at 450 °C. The chloroform molecule has a tetrahedral geometry.
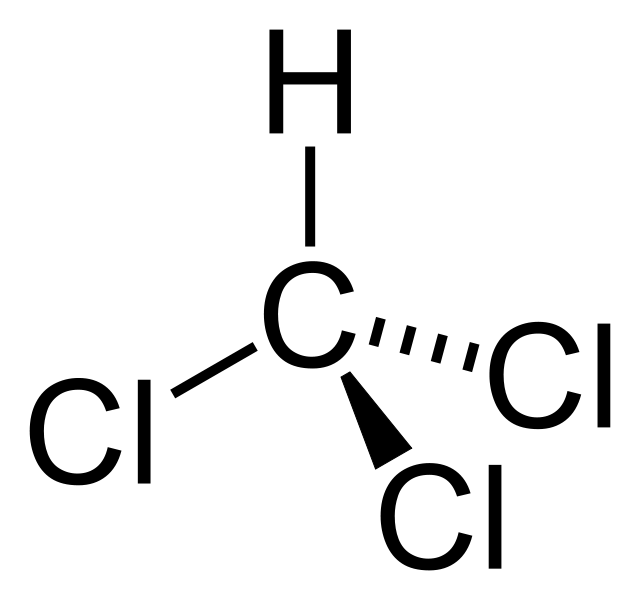
Figure 01: Chemical Structure of Chloroform
In industrial scale, we can produce this compound via heating a mixture of chlorine and chloromethane (or sometimes we use methane as well). Upon heating, a free radical halogenation occurs at 400–500 °C. There, the chlorinated compounds of chloromethane (or methane) forms which yield chloroform. There, this compound can undergo further chlorination, forming carbon tetrachloride. However, the end product of this reaction is a mixture of chloromethanes that we can separate via distillation in order to get chloroform.
There are many uses of chloroform. It is useful as a solvent because hydrogen atom in this molecule can undergo hydrogen bonding. We can use it as a reagent for many chemical reactions as well. Ex: as a source of dichlorocarbene group. More importantly, chloroform is well-known for its anaesthetic properties.
What is Carbon Tetrachloride?
Carbon tetrachloride is CCl4 , which we commonly call “tetrachloromethane”. It is a colourless liquid having a sweet smell. We can detect it from its smell even at low levels. In the cleaning industry, the common name of this compound is carbon tet.
The molar mass is 153.81 g/mol. The melting point is −22.92 °C, and the boiling point is 76.72 °C. The geometry of this molecule is tetrahedral geometry. Since it has four chlorine atoms bonded to a single carbon atom, the bond angles of the molecules are equal. We call it a “symmetrical geometry”. Due to this geometry, the compound is nonpolar. It resembles the structure of methane molecule which has four hydrogen atoms bonded to a single carbon atom.
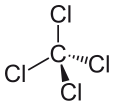
Figure 02: Chemical Structure of Carbon Tetrachloride
There are many uses of carbon tetrachloride. Before the prohibition, this compound was used to produce CFC in large scale. Nowadays, we do not produce CFC since it harms the ozone layer. Carbon tetrachloride is the key ingredient in lava lamps. Once it was a popular solvent, but now we don’t use it due to its adverse health effects. Moreover, we widely use it in fire extinguishers, as a precursor to refrigerants and as a cleaning agent.
What is the Difference Between Chloroform and Carbon Tetrachloride?
Chloroform is CHCl3 and is useful as a powerful anaesthetic. Carbon tetrachloride is CCl4, which we commonly call “tetrachloromethane” is not an anaesthetic. This is the key difference between chloroform and carbon tetrachloride. Moreover, according to the molecular structure, chloroform has five atoms; one carbon atom, one hydrogen atom, and three chlorine atoms, and the molecular geometry is tetrahedral asymmetric geometry. But, although the carbon tetrachloride too has five atoms, it has one carbon atoms and four chlorine atoms, and the molecular geometry is tetrahedral symmetric geometry. Furthermore, considering their properties, the molar mass of chloroform is 119.37 g/mol. It appears as a dense colourless liquid and has a heavy ethereal odour. Whereas, the molar mass of carbon tetrachloride is 153.81 g/mol. It appears as a colourless liquid and has a sweet smell. The below infographic presents more details on the difference between chloroform and carbon tetrachloride in tabular form.

Summary – Chloroform vs Carbon Tetrachloride
Since both chloroform and carbon tetrachloride resemble in their chemical structure and composition, most people misunderstand them to be the same compound. But, carbon tetrachloride has only carbon and chlorine atoms whereas chloroform has carbon, chlorine and hydrogen atoms. Furthermore, the key difference between chloroform and carbon tetrachloride is that we can use chloroform as a powerful anaesthetic, but we cannot use carbon tetrachloride as an anaesthetic.
Reference:
1. “Chloroform.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine. Available here
2. “Carbon Tetrachloride.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine. Available here
Image Courtesy:
1.”Chloroform displayed”By Benjah-bmm27, vectorized by Fvasconcellos (Public Domain) via Commons Wikimedia
2.”Tetrachlormethan”By NEUROtiker (talk) – Own work, (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vx6Wmq6eWpL%2BuecCnm2abkaevsLqMrZytqpGYta270aKbnmc%3D