Difference Between Carbene and Carbanion
Table of Contents
The key difference between carbene and carbanion is that the carbene has a divalent carbon atom, whereas the carbanion has a trivalent carbon atom.
Carbene and carbanion are organic molecules containing carbon and hydrogen or other atoms. These two types of organic compounds are different from each other in their shape, geometry, valency, oxidation state, charge, etc. So, this article addresses the difference between carbene and carbanion on these aspects.
CONTENTS
1. Overview and Key Difference
2. What is Carbene
3. What is Carbanion
4. Side by Side Comparison – Carbene vs Carbanion in Tabular Form
5. Summary
What is Carbene?
A carbene is an organic molecule having a neutral carbon atom with a valence of two. In other words, the carbon atom in a carbene compound contains two unshared valence electrons. The general chemical formula for this type of compound is R-(C:)-R’ or R=C: where the R and R’ represent substituents or hydrogen atoms. The general structure of this compound is as follows:

Figure 01: Chemical Structure of Carbene
Moreover, there are two classes of carbenes as singlet carbene and triplet carbene. The singlet carbene compounds are spin-paired compounds. These molecules adopt an sp2 hybrid structure. Triplet carbene compounds have unpaired electrons. Most carbene compounds have a triplet structure in their ground state, except for carbene compounds containing nitrogen, oxygen, or sulfur atoms.
We can call a carbene compound a singlet or a triplet structure depending on the electronic spins of these molecules. For example, triplet carbene compounds are paramagnetic, and we can observe this structure by electron spin resonance spectroscopy if we can make these molecules persist long enough for the analysis. Furthermore, the spin of singlet carbene molecules is zero while the spin of the triplet carbene molecules is one. In addition to these, singlet carbene molecules are stable in an aqueous medium, whereas triplet carbene molecules are stable in a gaseous state.
What is Carbanion?
A carbanion is an anion having a trivalent carbon atom and a net negative charge on the ion. A trivalent carbon atom is an ion that contains a carbon atom that has formed three covalent bonds. To name a compound as a carbanion, it should have a formal negative charge in at least one resonance structure.
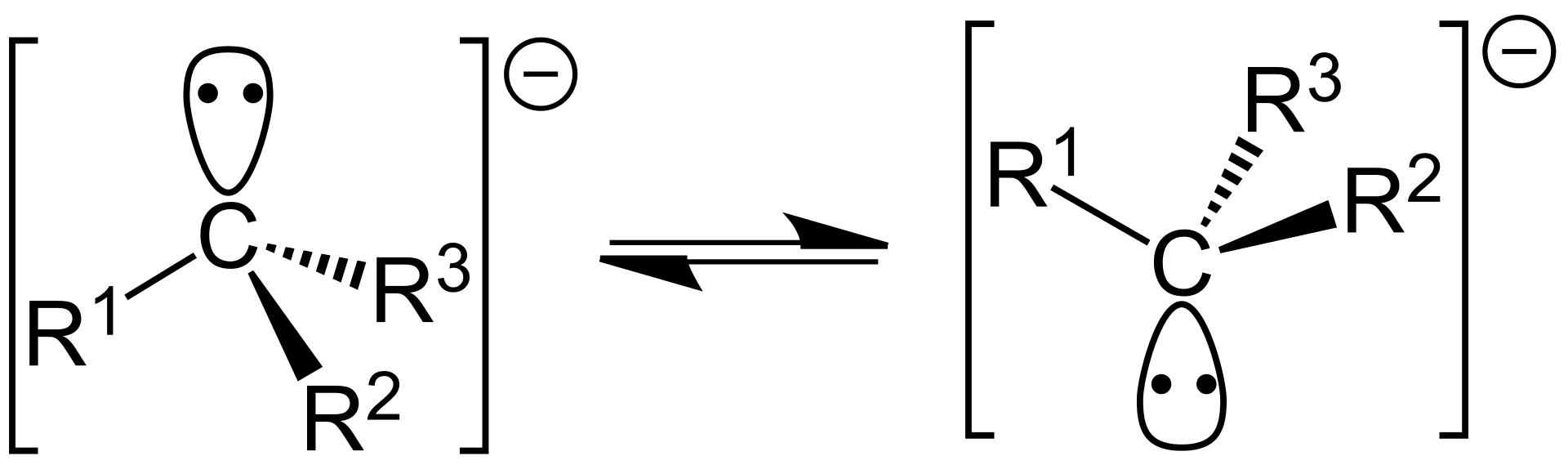
Figure 02: Resonance Structures of Carbanions
Moreover, a carbanion has no pi-electron delocalization and these molecules assume a trigonal pyramidal, bent, or linear geometry when the carbanion has three, two, or one substituent, respectively. More importantly, a carbanion is the conjugate base of a carbon acid.
Typically, carbanions are nucleophilic and basic. The nucleophilicity and the basicity of these compounds can be determined by the type of substituents on the carbon atom. Besides, there can be chiral carbanions as well.
Furthermore, the electron density on the negatively charged carbon atom in a carbanion makes the ion able to react with electrophiles having varying strengths including carbonyl groups, halogenated reagents, etc.
What is the Difference Between Carbene and Carbanion?
Carbene and carbanion are organic molecules containing carbon and hydrogen or other atoms. The key difference between carbene and carbanion is that carbene has a divalent carbon atom, whereas carbanion has a trivalent carbon atom. Therefore, carbene compounds have carbon atoms having two covalent chemical bonds while carbanion compounds have three covalent bonds on the carbon atom and a formal negative charge as well.
Below infographic summarizes the differences between carbene and carbanion.

Summary – Carbene vs Carbanion
Carbene and carbanion are organic molecules containing carbon and hydrogen or other atoms. The key difference between carbene and carbanion is that carbene has a divalent carbon atom, whereas carbanion has a trivalent carbon atom.
Reference:
1. “Carbenes.” Chemistry LibreTexts, 13 Sept. 2020, Available here.
Image Courtesy:
1. “Carbene” By Hbf878 – Own work, CC0) via Commons Wikimedia
2. “Carbanion Structural Formulae V.1” By Jü – Own work (CC0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vwKuZnqaVYq6vsIycmKuakaO2sLqO