Difference Between Calomel and Glass Electrode
Table of Contents
The key difference between calomel and glass electrode is that the calomel electrode is a type of reference electrode, whereas the glass electrode is a non-reference electrode.
An electrode is a small apparatus that is used to measure the pH of a system. Calomel electrode or saturated calomel electrode (SCE) is used mainly as a reference electrode that is useful in calibrating other electrodes. In contrast, the glass electrode is not a reference electrode, and it is used along with some other reference electrodes.
CONTENTS
1. Overview and Key Difference
2. What is a Calomel Electrode
3. What is a Glass Electrode
4. Side by Side Comparison – Calomel vs Glass Electrode in Tabular Form
5. Summary
What is a Calomel Electrode?
A calomel electrode or saturated calomel electrode is a type of reference electrode that uses a system of mercury and its ionized species to measure the potential. The electrode is composed of mercury and mercuric chloride.
Mercury is in the form of liquid elemental state while mercuric chloride is in the form of a paste. These two components are attached to a rod that is immersed in a saturated solution of potassium chloride. Here, it is a necessity to have a saturated solution because then the activity of the solution is fixed by potassium chloride, allowing the electrode to have a voltage lower or closer to the voltage of the saturated hydrogen electrode. Also, the saturated solution allows the exchange of chloride ions through a salt bridge.
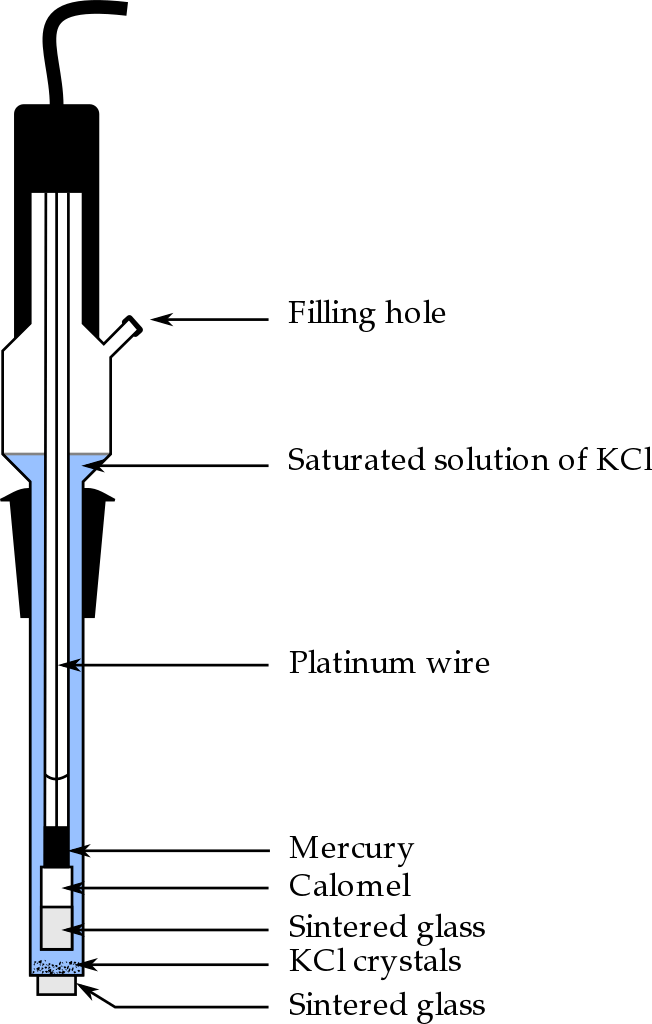
Figure 01: Calomel Electrode
There are few applications of calomel electrode, including pH measurement, cyclic voltammetry, and aqueous electrochemistry. The silver/silver chloride electrode is another reference electrode that works the same way as the calomel electrode. Since the calomel electrode contains mercury, which has a great health hazard compared to silver, it is replaced by the silver/silver chloride reference electrode.
What is a Glass Electrode?
A glass electrode is a type of non-ion selective electrode that contains a doped glass membrane. This electrode is sensitive to a specific ion in the analyte. Most commonly, this electrode is used in measuring pH, where the electrode is sensitive to the hydrogen ion.
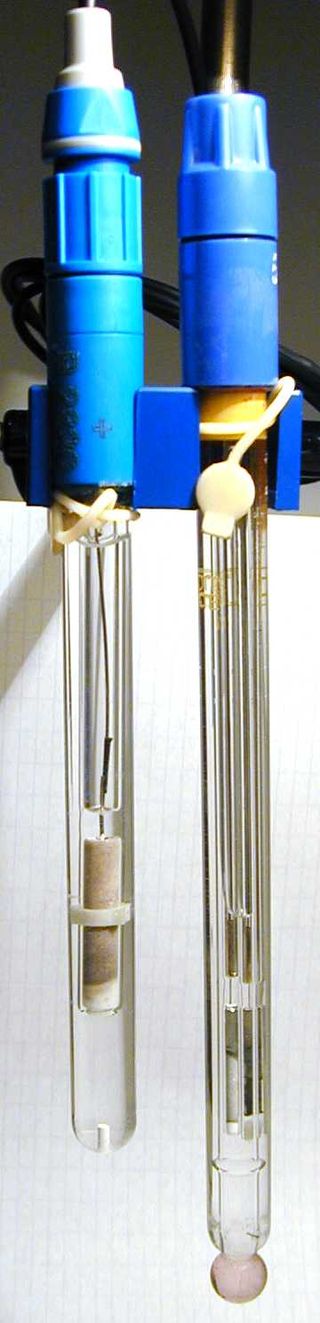
Figure 02: A Silver/Silver Chloride Reference Electrode (left) and a Glass Electrode (right)
Almost all glass electrodes respond to a single ion such as hydrogen ion, sodium ion, silver ion, etc. The most common glass electrode is the pH electrode. However, there are some other electrodes which are sensitive to lead ion and cadmium ion.
What is the Difference Between Calomel and Glass Electrode?
Calomel electrode and glass electrode are two types of electrodes used for the measurement of the pH of a system. However, the key difference between calomel and glass electrode is that calomel electrode is a type of reference electrode, whereas the glass electrode is a non-reference electrode. Besides, the calomel electrode is used for pH measurement, cyclic voltammetry, aqueous electrochemistry, etc. while the glass electrode is mainly used for pH measurements.
Below is a summary of the difference between calomel and glass electrode.
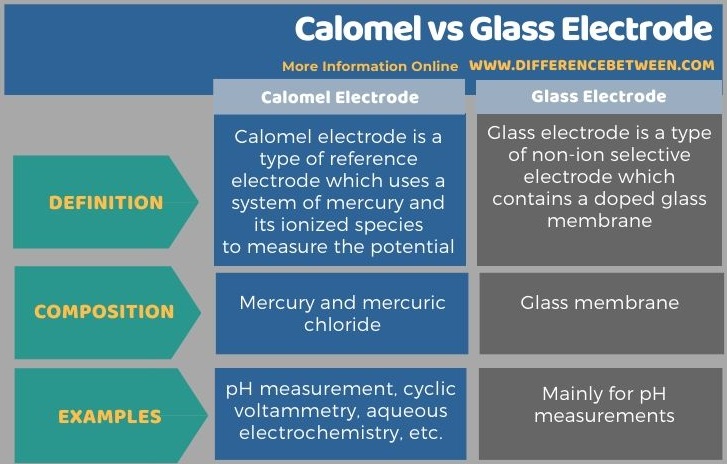
Summary – Calomel vs Glass Electrode
Electrodes are small apparatus which can be used in measuring the pH of a system. Calomel electrode and glass electrode are such two types of electrodes. The key difference between calomel and glass electrode is that calomel electrode is a type of reference electrode, whereas the glass electrode is a non-reference electrode.
Reference:
1. “Standard Electrodes.” Chemistry LibreTexts, Libretexts, 5 June 2019, Available here.
2. “Saturated Calomel Electrode.” Wikipedia, Wikimedia Foundation, 20 Mar. 2020, Available here.
3. “Glass Electrode.” Wikipedia, Wikimedia Foundation, 11 May 2020, Available here.
Image Courtesy:
1. “ECS electrode” By Bachi-Bouzouk – Own work (CC0) via Commons Wikimedia
2. By No machine-readable author provided. Nolf assumed (based on copyright claims). – No machine-readable source provided. Own work assumed (based on copyright claims) (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vwKWmpp2cYq6vsIygo5qro2KyrbHCramonJVk