Difference Between Calcium Lactate and Calcium Carbonate
Table of Contents
The key difference between calcium lactate and calcium carbonate is that the calcium lactate contains two lactate ions for each calcium ion whereas the calcium carbonate contains one carbonate ion per each calcium ion. Furthermore, they both differ in the application too.
Both calcium lactate and calcium carbonate are inorganic salts. Both these compounds are useful as calcium supplements to treat low blood levels of calcium for people who do not get enough calcium from their diet. Let us discuss more details about these compounds and thereby distinguish the difference between calcium lactate and calcium carbonate.
CONTENTS
1. Overview and Key Difference
2. What is Calcium Lactate
3. What is Calcium Carbonate
4. Side by Side Comparison – Calcium Lactate vs Calcium Carbonate in Tabular Form
5. Summary
What is Calcium Lactate?
Calcium lactate is an inorganic salt having the chemical formula C6H10CaO6. It contains two lactate ions per each calcium cation. The molar mass is 218.22 g/mol, and it appears as a white or off-white powder. Its melting point is 240 °C. Moreover, the lactate anion has chirality; thus, it has D and L isomers. Usually, living organisms synthesise and metabolise the L isomer. However, some bacteria can synthesize the D isomer as well. Furthermore, this compound forms several hydrates; the most common hydrate is pentahydrate form.
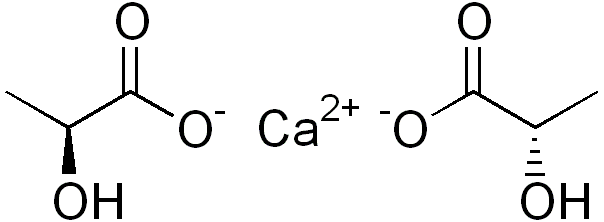
Figure 01: Chemical Structure of Calcium Lactate
We can produce calcium lactate via the reaction of lactic acid with calcium carbonate (or calcium hydroxide). In industrial-scale production, the common production strategy is fermentation of carbohydrates in the presence of calcium carbonate or hydroxide.
The major applications of this compound are in medicine; it is used as an antacid. Moreover, it is useful to treat hypocalcaemia (the medical term for calcium deficiency). We don’t have to take this compound with food because our body can absorb this compound at various pH values. In addition to that, we can find this compound in various mouthwashes as well.
What is Calcium Carbonate?
Calcium carbonate is an inorganic salt having the chemical formula CaCO3. Therefore, it contains one carbonate anion per one calcium cation. The molar mass is 100 g/mol, and it appears as a fine white powder with a chalky taste. The melting point is 1,339 °C, and it has no boiling point since it undergoes decomposition at high temperatures.
When considering the occurrence of this salt, it exists on the earth’s crust as calcium minerals such as calcite, aragonite, etc. Egg shells, snail shells and sea shells are the biological sources. Moreover, we can prepare this compound via mining or quarry the mineral mentioned above deposits. Alternatively, we can produce it via reacting calcium oxide with water; this gives calcium hydroxide. Afterwards, we have to pass carbon dioxide through this product to get calcium carbonate.

Figure 02: Chemical Structure of Calcium Carbonate
The major applications of this compound are mainly in the construction industry, where it is important as a building material. Therefore, it is a common ingredient in cement. Moreover, it is the main component in blackboard chalk. There are health and dietary applications as well. It is an inexpensive dietary calcium supplement. In addition to that, we can use it as a phosphate binder to treat hyperphosphatemia. Apart from that, it is useful as a filler for tablets in the pharmaceutical industry.
What is the Difference Between Calcium Lactate and Calcium Carbonate?
Calcium lactate is an inorganic salt having the chemical formula C6H10CaO6. It contains two lactate ions for each calcium ion. Among important chemical data, the molar mass of this compound is 218.22 g/mol and the melting point is 240 °C. Moreover, calcium lactate is useful as an antacid, to treat hypocalcaemia, as an ingredient in mouth washes and as a food additive as well. Calcium carbonate, on the other hand, is an inorganic salt having the chemical formula CaCO3. It contains one carbonate ion per each calcium ion. The molar mass is 100 g/mol and the melting point is 1,339 °C. In addition to that, it is useful as a building material, as blackboard chalk, as an inexpensive dietary calcium supplement, etc. The below infographic presents more details on the difference between calcium lactate and cacalcium carbonate.
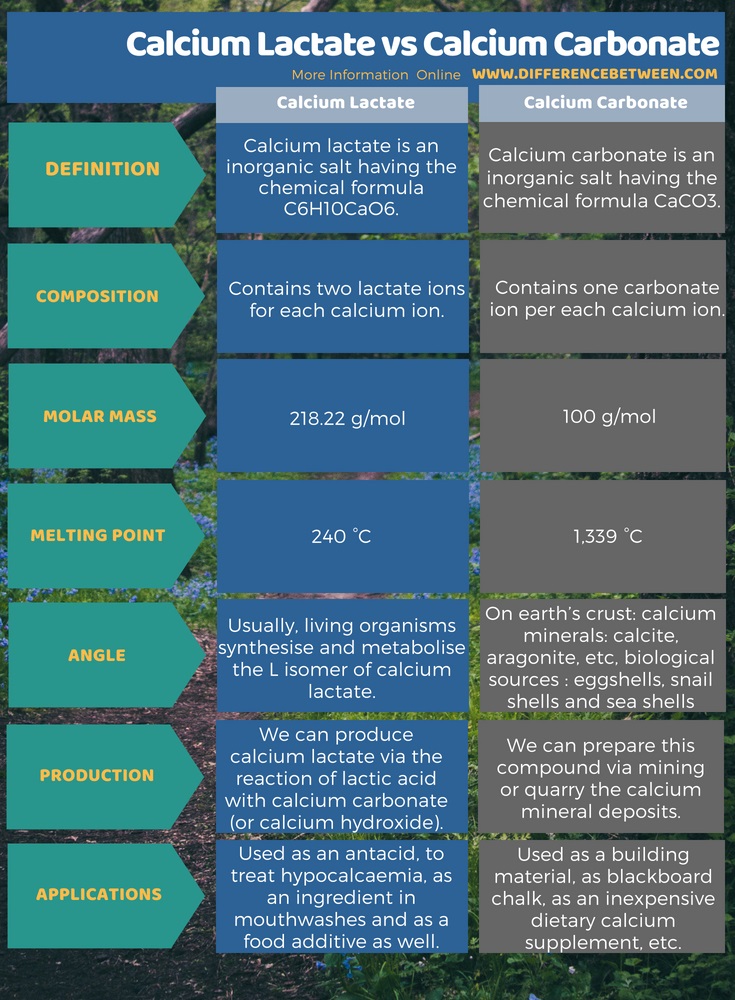
Summary – Calcium Lactate vs Calcium Carbonate
Both calcium lactate and calcium carbonate are inorganic salts of calcium. The difference between calcium lactate and calcium carbonate is that calcium lactate contains two lactate ions for each calcium ion whereas calcium carbonate contains one carbonate ion per each calcium ion.
Reference:
1. “Calcium Lactate Oral : Uses, Side Effects, Interactions, Pictures, Warnings & Dosing.” WebMD, WebMD. Available here
2. “Calcium Carbonate.” Wikipedia, Wikimedia Foundation, 27 July 2018. Available here
Image Courtesy:
1.’Calcium lactate’By Edgar181 – Own work, (Public Domain) via Commons Wikimedia
2.’Calcium carbonate’By Edgar181 (talk) – Own work, (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vwKWaoq2dYrmir9Oaq55lkaOxbq%2FApZqirZ1isKK%2BwailmqyVZA%3D%3D