Difference Between Benzonase and DNase
Table of Contents
Key Difference – Benzonase vs DNase
Degradation of nucleic acids is important for many molecular biology techniques. It is widely used in recombinant DNA technology to get rid of unwanted fragments of DNA and RNA. Nucleic acid degrading enzymes are referred to as Nucleases, and they can be of different types based on the required function. Nucleases that degrade DNA are known as DNases whereas the ones that degrade RNA are known RNases. These enzymes are mostly used in in vitro experiments where in vitro molecular tests are carried out to isolate pure DNA, RNA or proteins. Benzonases are a type of nucleases that degrade both DNA and RNA whereas DNases degrade only DNA. This is the key difference between Benzonase and DNase.
CONTENTS
1. Overview and Key Difference
2. What is Benzonase
3. What is DNase
4. Similarities Between Benzonase and DNase
5. Side by Side Comparison – Benzonase vs DNase in Tabular Form
6. Summary
What is Benzonase?
Benzonase is is a genetically engineered endonuclease from Serratia marcescens. This enzyme is produced in E. coli hosts in industrial scale. Benzonase is capable of cleaving double-stranded DNA, linear DNA, circular DNA and single-stranded RNA. Thus Benzonase is commercially important. Benzonase enzyme is a protein dimer which has 245 identical amino acids, ~30 kDa subunits with two essential disulfide bonds. Benzonase cleaves nucleic acids at its 5’ end and results in fragments with free 5’ends. Benzonase can cleave nucleic acids at any sequence but prefers GC rich regions.
Benzonase is stored at -20 0C. The optimum pH for enzyme activity is found to be 8.0 – 9.2. The applications of Benzonase include sample preparation for protein 2D gel electrophoresis where Benzonase removes bound nucleic acids and removal of nucleic acid contaminants from recombinant protein preparations. It is also used to reduce the viscosity of protein extracts and prevent clumping of cells in a cell mixture.
What is DNase?
DNase is a nuclease, hydrolytic enzyme which is only capable of cleaving double-stranded DNA. There are two main types of DNases: DNase I and DNase II. DNase I participates in cleaving double-stranded DNA to produce polynucleotides with 5’ free ends. DNase II is involved in cleaving double-stranded DNA to produce polynucleotide strands with 3’ free ends or overhangs.
DNase I
DNase I functions at an optimum pH between 7.0 – 8.0. The enzyme activity depends on many ionic cofactors which include, Ca2+, Mg2+ or Mn2+. The activity of Mg2+ and Mn2+ decides the function of the DNase I. In the presence of Mg2+, DNase I cleaves each strand of dsDNA independently. This takes place in a random manner. In contrast, in the presence of Mn2+, the enzyme cleaves both DNA strands at approximately the same site. This cleavage will result in producing two types of DNA fragments; one type with blunt ends and another type with one or two nucleotide overhangs.
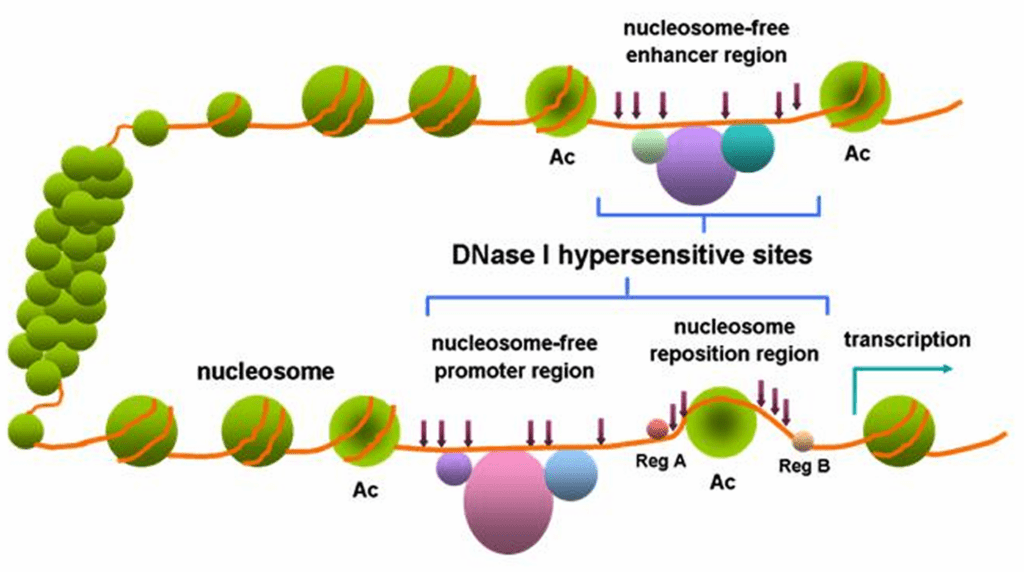
Figure 02: DNase
DNase II
DNase II functions at an optimum pH of 4.5-5.0 and divalent metal ions are required for its activity, similar to DNase I. The mechanism of DNase II is known to consist of three main steps.
The main inhibitors of DNase enzyme include metal chelators, transition metals and chemicals such as Sodium dodecyl sulfate and β – mercaptoethanol.
The main applications of DNase include preparation of DNA-free RNA extracts and protein extracts, and removal of template DNA during in vitro transcription experiments.
What are the Similarities Between Benzonase and DNase?
- Both are hydrolytic enzymes.
- Both are nucleases.
- Both participate by cleaving the phosphodiester bonds of nucleic acids.
- Both require an optimum pH and storage temperatures to maintain the activity of the enzyme.
- Inhibitors of enzymes include chelating agents, transition metals, and detergent chemicals.
- Applications are mainly focused on obtaining high purity extracts of DNA, RNA, and proteins.
- Both enzymes can be produced via genetic engineering.
What is the Difference Between Benzonase and DNase?
Benzonase vs DNase | |
| Benzonase is an enzyme that is capable of cleaving double-stranded DNA, linear DNA, circular DNA, and RNA. | DNase is an enzyme which is capable of cleaving double-stranded DNA. |
| Substrate for the Enzyme | |
| Both DNA and RNA are substrates for benzonase. | DNA is the substrate for DNase. |
| Structure | |
| Optimum pH range of Benzonase is 7.0 -8.0 | Optimum pH ranges of DNase I is 7.0 – 8.0 and DNase II is 4.5 – 5.0. |
Summary – Benzonase vs DNase
Nuclease enzymes are widely used in different experimental procedures with regard to molecular biology and genetic engineering. Benzonase and DNase are two types of nucleases. Benzonase is involved in degrading both DNA and RNA whereas DNase is involved in cleaving double-stranded DNA. This is the basic difference between benzonase and DNase. At present, both these nuclease types are produced through recombinant DNA technology which yields a higher quality enzymes that are optimized for maximum production.
Download PDF Version of Benzonase vs DNase
You can download PDF version of this article and use it for offline purposes as per citation note. Please download PDF version here Difference Between Benzonase and DNase
References:
1.“Deoxyribonuclease I from bovine pancreas D5025.” Sigma-Aldrich, Available here. Accessed 19 Sept. 2017.
2. “Deoxyribonuclease II.” Deoxyribonuclease II – Worthington Enzyme Manual, Available here. Accessed 19 Sept. 2017.
Image Courtesy:
1.”DNAse hypersensitive site” By Wang Y-M, Zhou P, Wang L-Y, Li Z-H, Zhang Y-N, et al. – Wang Y-M, Zhou P, Wang L-Y, Li Z-H, Zhang Y-N, et al. (2012) Correlation Between DNase I Hypersensitive Site Distribution and Gene Expression in HeLa S3 Cells. PLoS ONE 7(8): e42414. doi:10.1371/journal.pone.0042414 (CC BY-SA 2.5) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26uxKexqKaRqLJurc2dZK%2BrXZm7or%2FEaA%3D%3D