Difference Between Anionic and Cationic Polymerization
Table of Contents
Key Difference – Anionic vs Cationic Polymerization
Anionic polymerization and cationic polymerization are two types of chain growth polymerization reactions which are used to synthesize various types of polymers. Both these reactions have the same reaction mechanism, but the reaction initiator is different. Anionic polymerization reactions are initiated by an active anionic species, whereas the cationic polymerization reactions are initiated by an active cationic species. This is the key difference between anionic and cationic polymerization. Both these polymerization reactions are sensitive to the solvent used.
What is Anionic Polymerization?
Anionic polymerization is a chain growth reaction which begins by an anion. Several different types of initiators are used in anionic polymerization. This series of reactions takes place in three steps: initiation, chain propagation, and chain termination. These polymerization reactions are initiated by nucleophilic addition to the double bond of the monomer. Therefore, the initiator used in the reaction should be a nucleophile.
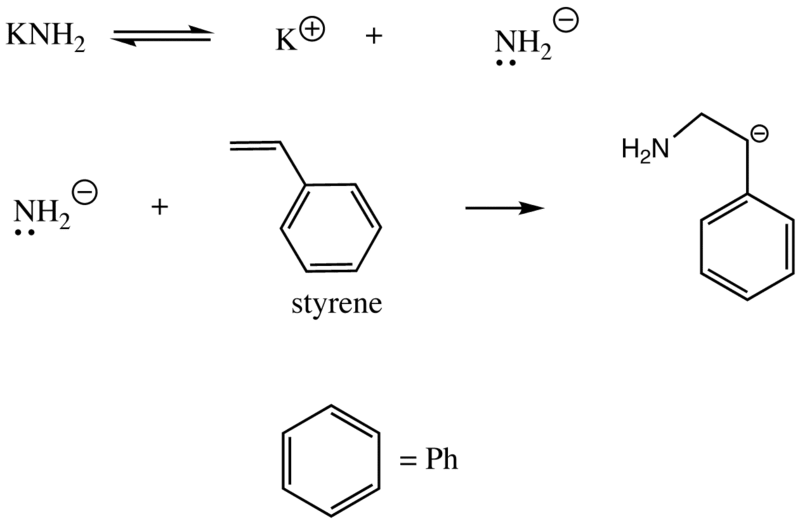
Initiation through strong anion
What is Cationic Polymerization?
Cationic polymerization can be considered as another category of chain growth polymerization reactions. A cation initiates this reaction by transferring its charge to a monomer, which then results in producing a more reactive species. Next, the reactive monomer reacts similarly with other monomers to form a polymer. There are only a limited number of monomers which can facilitate the cationic polymerization chain reaction. Olefins containing electron-donating substituents and heterocycles are suitable for these types of reactions.
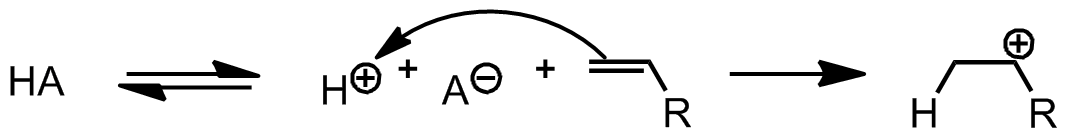
Initiation by protic acids
What is the difference between Anionic and Cationic Polymerization?
Examples of Initiators and Monomers:
Monomers:
Anionic Polymerization: Anionic polymerization takes place with monomers having electron-withdrawing groups such as nitrile, carboxyl, phenyl, and vinyl.
Cationic Polymerization: Alkenes having alkoxy, phenyl, vinyl and 1,1-dialkyl substituents are some examples of monomers used in the cationic polymerization.
Initiators:
Anionic Polymerization: Nucleophiles such as hydroxide, alkoxide, cyanide or a carbanion can act as initiators in anionic polymerization. The carbanion can originate from organometallic species such as alkyl lithium or Grignard reagent.
Cationic Polymerization: Electrophilic agents such as halohydric acids (HCl, HBr, H2SO4, HClO4) are one group of initiators used in the cationic polymerization reactions. In addition, lewis acids (electron acceptors) and compounds capable of generating carbonium ions can also initiate polymerization. Examples of Lewis acids are AlCl3, SnCl4, BF3, TiCl4, AgClO4, and I2. However, lewis acids require a co-initiator such as H2O or an organic halogen compound.
Mechanism:
Anionic Polymerization: Anionic polymerization requires an initiator to start the reaction and a monomer to form the polymer. In this case, a reactive anionic species initiates the reaction by reacting with a monomer. The resulting monomer is a carbanion, which then reacts with another monomer to form a new carbanion. The reaction proceeds by adding a monomer to the growing chain in the same way, and this produces the polymer chain. This is called “chain propagation.”
Cationic Polymerization: A reactive cationic species initiates the reaction by binding and transferring its charge to a monomer. The resulting reactive monomer then reacts with another monomer to form a polymer in the same way as in anionic polymerization.
Reaction Rate:
Anionic Polymerization: The rate of the anionic polymerization reactions is relatively slower than the cationic polymerization reactions because the negative charge on the anionic initiator can be stabilized by several other factors. When these ions are stable, they will become less reactive.
Cationic Polymerization: The rate of the cationic polymerization reactions is relatively faster than anionic polymerization reactions because the cationic initiator is very reactive, difficult to control and stabilize.
Applications:
Anionic Polymerization: Anionic polymerization is used to manufacture some important materials such as polydiene synthetic rubbers, solution styrene/butadiene rubbers (SBR), and styrenic thermoplastic elastomers.
Cationic Polymerization: Cationic polymerization is used in the production of polyisobutylene (used in inner tubes) and poly (N-vinylcarbazole) (PVK).
References: “Cationic Polymerization”. Wikipedia. “Anionic addition polymerization”. Wikipedia “Catonic Vinly Polymerization” Polymerscience Learning Center “Polymerization Reactions”. Bodner Research Lab Image Courtesy: “Protic acid initiation” By MatChem121 – Own work (CC BY 3.0) via Commons Wikimedia “AAP Init Strong Anion” By Chem538grp1w09 – Own work (Public Domain) via Commons WikimediancG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26tzaKmp6GTYq6vsIyvqmabkam2sLrInGSpp5yuuqa%2ByLOYraGfo3w%3D