Difference Between Alkanes and Alkenes
Table of Contents
Key Difference – Alkanes vs Alkenes
Alkanes and Alkenes are two types of hydrocarbon families which contain carbon and hydrogen in their molecular structure. The key difference between Alkanes and Alkenes is their chemical structure; alkanes are saturated hydrocarbons with the general molecular formula of CnH2n+2 and alkenes are said to be an unsaturated hydrocarbon group since they contain a double bond between two carbon atoms. They have the general molecular formula of CnH2n.
What are Alkanes?
Alkanes contain only single bonds between Carbon and hydrogen atoms (C-C bonds and C-H bonds). Therefore, they are called “saturated hydrocarbons”. According to the orbital hybridization model, all the carbon atoms in Alkenes have the SP3 hybridization. They form sigma bonds with Hydrogen atoms, and the resulting molecule has the geometry of a tetrahedron. Alkanes can be sub-divided into two groups according to their molecular arrangements; acyclic alkanes (CnH2n.+2) and cyclic alkanes (CnH2n).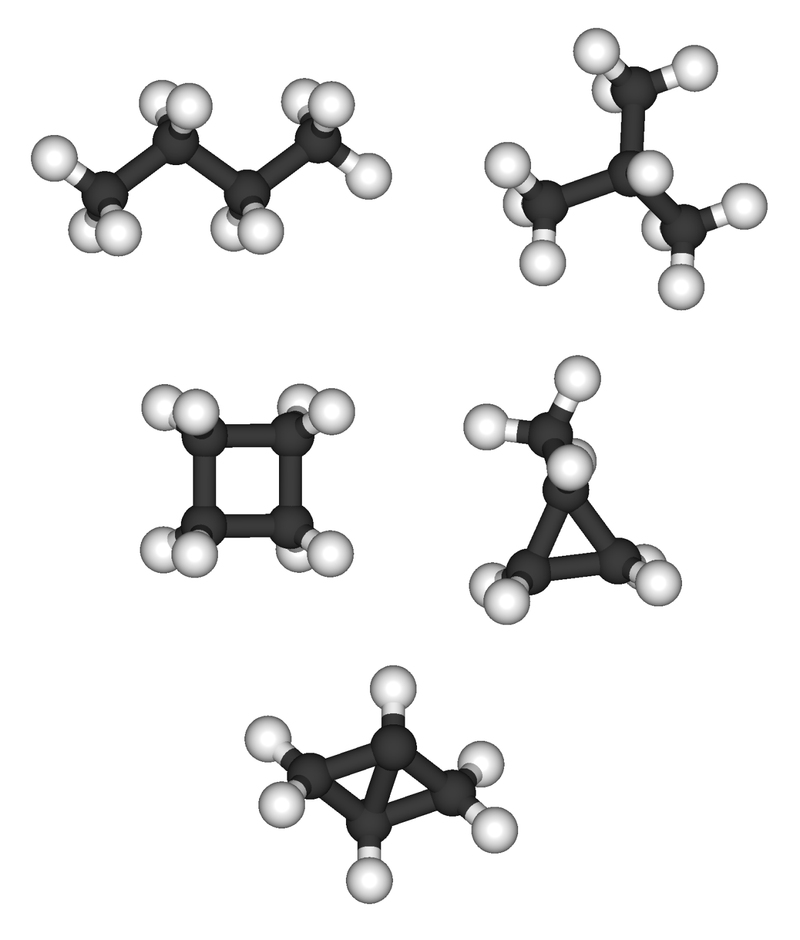
What are Alkenes?
Alkenes are the hydrocarbons, containing a Carbon-Carbon (C=C) double bond. “Olefins” is the old name used to refer to the alkene family. The smallest member of this family is ethane (C2H4); it was called olefiant gas (In Latin: ‘oleum’ means ‘oil’ + ‘facere’ means ‘to make’) in early days. This is because the reaction between C2H4 and Chlorine gives C2H2Cl2, oil.
What is the difference between Alkanes and Alkenes?
Chemical Structure of Alkanes and Alkenes
Alkanes: Alkanes have the general molecular formula CnH2n+2. Methane (CH4) is the smallest alkane.
| Name | Chemical formula | Acyclic structure |
| Methane | CH4 | CH4 |
| Ethane | C2H6 | CH3CH3 |
| Propane | C3H8 | CH3CH2CH3 |
| Butane | C4H10 | CH3CH2CH2CH3 |
| Pentane | C5H12 | CH3CH2CH2CH2CH3 |
| Hexane | C6H14 | CH3CH2CH2 CH2CH2CH3 |
| Heptane | C7H16 | CH3CH2CH2CH2CH2CH2CH3 |
| Octane | C8H18 | CH3 CH3CH2CH2CH2CH2CH3CH3 |
Alkenes: Alkenes have the general chemical formula of CnH2n. Alkenes are considered to be unsaturated hydrocarbons since they do not contain the maximum number of Hydrogen atoms that can be owned by a hydrocarbon molecule.
| Name | Chemical formula | Structure |
| Ethene | C2H4 | CH2 = CH2 |
| Propene | C3H6 | CH3CH = CH2 |
| Butene | C4H8 | CH2=CHCH2CH3, CH3CH=CHCH3 |
| Pentene | C5H10 | CH2=CHCH2CH2CH3, CH3CH=CHCH2CH3 |
| Hexene | C6H12 | CH2=CHCH2 CH2CH2CH3CH3CH=CHCH2CH2CH3 CH3CH2CH=CHCH2CH3 |
| Heptene | C7H14 | CH=CHCH2CH2CH2CH2CH3CH3CH=CH2CH2CH2CH2CH3 |
Chemical Properties of Alkanes and Alkenes
Alkanes:
Reactivity:
Alkanes are inert to many chemical reagents. This is because Carbon–Carbon (C-C) and Carbon – Hydrogen (C-H) bonds are quite strong since Carbon and Hydrogen atoms have nearly the same electronegativity values. Therefore, it is very difficult to break their bonds, unless they are heated to fairly high temperatures.
Combustion:
Alkanes can readily burn in the air. The reaction between Alkanes with excess Oxygen is called “combustion”. In this reaction, alkanes convert to Carbon dioxide (CO2) and water.
CnH2n + (n + n/2) O2 → n CO2 + nH2O
C4H10 + 13/2 O2 → 4 CO2 + 5H2O
Butane Oxygen Carbon Dioxide Water
The combustion reactions are exothermic reactions (they give off heat). As a result, alkanes are used as a source of energy.
Alkenes:
Reactivity:
Alkenes react with Hydrogen in the presence of a finely divided metal catalyst to form the corresponding alkane. The rate of the reaction is very low without a catalyst.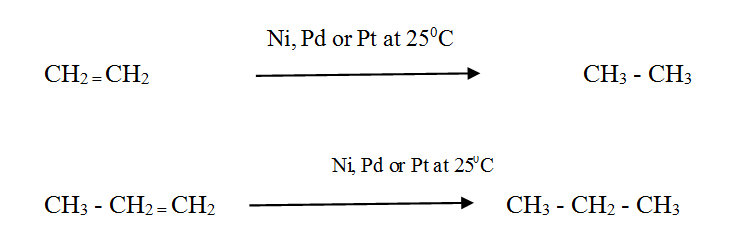
Catalytic hydrogenation is used in food industry to convert liquid vegetable oils to semi-solid fat in making margarine and solid cooking fat.
Physical Properties of Alkanes and Alkenes
Forms
Alkanes: Alkanes exist as gases, liquids and solids. Methane, ethane, propane and butane are gases at room temperature. The unbranched structures of hexane, pentane and heptane are liquids. Alkanes which have a higher molecular weight are solids.
CH4 to C4H10 are gases
C5H12 to C17H36 are liquids, and
Alkanes with higher molecular weight are soft solids
Alkenes: Alkenes show similar physical properties of the corresponding Alkane. Alkenes which have lower molecular weights (C2H4 toC4H8) are gases at room temperature and atmospheric pressure. Alkenes having a higher molecular weight are solids.
Solubility:
Alkanes: Alkanes do not dissolve in water. They are dissolved in non-polar or weakly polar organic solvents.
Alkenes: Alkenes are relatively polar molecules due to the C=C bond; therefore, they are soluble in non-polar solvents or solvents of low polarity. Water is a polar molecule and alkenes are slightly soluble in water.
Density:
Alkanes: The densities of Alkanes are lower than the density of water. Their density value is nearly 0.7 g mL-1, considering the density of water as 1.0 g mL-1.
Alkenes: The densities of Alkenes are lower than the density of water.
Boiling points:
Alkanes: The boiling point of unbranched alkanes smoothly increases as the number of Carbon atoms and the molecular weight are increasing. In general, branched alkanes have lower boiling points compared to the unbranched alkanes, having the same number of Carbon atoms.
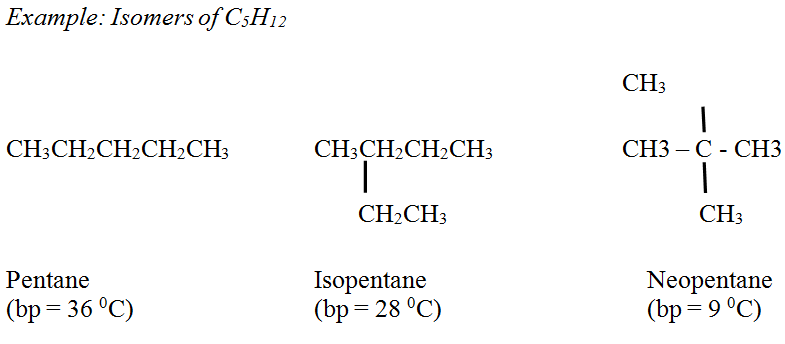
Alkenes: Boiling points are similar to the corresponding alkanes with a small variation.
References: CliffsNotes. (n.d.). Retrieved July 06, 2016, from here. Zum Directory-modus. (n.d.). Retrieved July 06, 2016, from here Polarity. (n.d.). Retrieved July 06, 2016, from here Dipole moments. (2013). Retrieved July 06, 2016, from here Structural isomer. (n.d.). Retrieved July 06, 2016, from here An introduction to alkenes. (n.d.). Retrieved July 06, 2016, from here Image Courtesy: “Alkene names” By Chris Evans – (CC0) via Commons Wikimedia “Saturated C4 hydrocarbons ball-and-stick” By Fvasconcellos – Own work (Public Domain) via Commons WikimediancG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26ty6SYp52jYq6vsIyvqmaZnKCyr7HSaA%3D%3D