Difference Between Acidity and Alkalinity of Water
Table of Contents
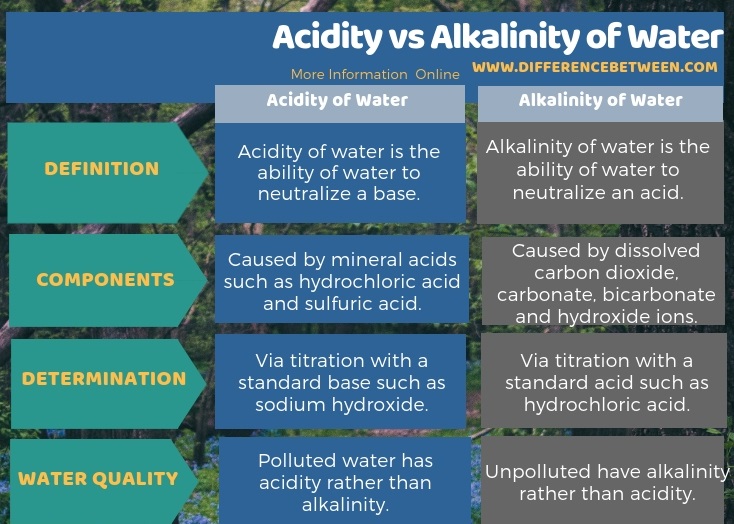
The key difference between acidity and alkalinity of water is that the acidity of water is the water’s ability to neutralize a base whereas the alkalinity of water determines water’s ability to neutralize an acid.
We can name acidity of water as the “base neutralizing capacity” and alkalinity of water as the “acid neutralizing capacity”. We can determine these two parameters using laboratory experiments. It is very helpful in determining the water quality and the degree of water pollution.
CONTENTS
1. Overview and Key Difference
2. What is Acidity of Water
3. What is Alkalinity of Water
4. Side by Side Comparison – Acidity vs Alkalinity of Water in Tabular Form
5. Summary
What is Acidity of Water?
Acidity of water is the ability of water to neutralize a base. We can name it as the base neutralizing capacity of water. It also gives how much of a standard base that we need to add to water in order to raise the pH of water to a specific value. The major basic chemical species in water are hydroxide ions. Hence, the term acidity can also be given as the water’s capacity to neutralize hydroxide ions, though it is not very accurate because there can be some other basic chemical species as well.
The acidity of water arises due to the dissolution of mineral acids such as sulfuric acid and hydrochloric acid. Or else, the acidity of water can be a result of the dissolution of carbon dioxide gas. In drinking water sources, carbon dioxide is the major contributor to the acidity of water. If water has high acidity, the corrosiveness of water is also high. Therefore, it can harm water pipes made of copper. Hence, high levels of copper and lead may exist in acidic drinking water.

Figure 01: Endpoint for the Titration of a water sample for Acidity in the presence of Phenolphthalein
Generally, we can determine the acidity of water via titration with sodium hydroxide to any acceptable pH value. Phenolphthalein is an acid-base indicator that we use in this titration. Since this indicator gives its colour change at pH 8.3, we can titrate our water sample to this pH value. However, before starting the experiment, we should observe the colour of the indicator before adding any sodium hydroxide because our water sample may have the alkaline colour of the indicator if the water is alkaline. Then there is no use of determining the acidity of water since the acidity is zero.
What is Alkalinity of Water?
Alkalinity of water is the ability of water to neutralize an acid. The major cause for the alkalinity of water is salts of weak acids. Also, the principal chemical species that contribute to the alkalinity are hydroxide and bicarbonate. Most of the times, if water is unpolluted, we can observe alkalinity rather than acidity. This is because, all natural waters have dissolved carbon dioxide which eventually forms alkaline chemical species such as carbonate and bicarbonate. Hence, alkalinity of water is a good indicator that gives the total dissolved inorganic carbon in the water sample.

Figure 02: Carbonate Species that contribute to Alkalinity of Water
Besides, we can determine the alkalinity of water by determining how much acid we need to add to a water sample in order to decrease the pH of the water to a certain value. We can do the experiment via titration of water with an acid such as hydrochloric acid.
What is the Difference Between Acidity and Alkalinity of Water?
To begin with, the fundamental difference between acidity and alkalinity of water is that the Acidity of water is the ability of water to neutralize a base whereas alkalinity of water is the ability of water to neutralize an acid. Most of the times, unpolluted or natural water shows alkalinity rather than acidity because of the dissolved carbon dioxide. Therefore, polluted water has a high acidity.
Another important difference between acidity and alkalinity of water is that the contributors to acidity of water are mineral acids such as hydrochloric acid and sulfuric acid while for alkalinity it includes dissolved carbon dioxide, carbonate, bicarbonate and hydroxide ions.
The below infographic shows the difference between acidity and alkalinity of water in more detail, and in tabular form.
Summary – Acidity vs Alkalinity of Water
Acidity and alkalinity of water are good indicators of water quality because most of the natural water sources have alkalinity rather than an acidity. But, the key difference between acidity and alkalinity of water is that the acidity of water is the water’s ability to neutralize a base whereas the alkalinity of water determines water’s ability to neutralize an acid.
Reference:
1. “Dr. Darrin Lew.” Effects of Herbicides and Pesticides on Aquatic Life – Plant Adaptation. Available here
2. Admin. “Water Quality: PH and Alkalinity.” Center for Agriculture, Food and the Environment, 23 Jan. 2017. Available here
Image Courtesy:
1.”Phenolphthalein in Flask”By 384 – Own work, (CC BY-SA 4.0) via Commons Wikimedia
2.”Carbonate Bjerrum”By R Ge B – Own work, (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26twqKboqypYq6vsIyao6SZnJ67qsDYZqafZaeWwaa%2Bjg%3D%3D